
Scotland’s Biodiversity Progress to 2020 Aichi Targets: Conserving Genetic Diversity – Development of a national approach for addressing Aichi Biodiversity Target 13 that includes wild species
This report outlines a national approach for the conservation of genetic diversity suited to Scotland and applicable internationally. To achieve this, we have identified a set of criteria for defining terrestrial and freshwater species of socio-economic importance in Scotland, and selected an initial list of 26 species.
This list includes species of importance using the following criteria:
- National conservation priority wild species;
- Species of national cultural importance;
- Species providing key ecosystem services;
- Species of importance for wild harvesting (food and medicine);
- Economically important game species.
Its application is not restricted to Aichi Target 13 as the approach is designed as a generic scorecard for genetic diversity. It is thus relevant to post-2020 Convention on Biological Diversity targets focusing on genetic diversity.
Acknowledgements
We are grateful to the SEFARI Gateway ‘Think Tank’ fund for supporting this project, and for wider funding support from the Rural and Environmental Science and Analytical Services Division of Scottish Government and the Natural Environment Research Council. Kalani Seymour, Threatened Plants Project Manager for Plant Heritage provided extremely useful advice on horticultural genetic conservation
Contributors
Hollingsworth PM1*; O’Brien D2*; Ennos RA3; Yahr R1; Neaves L1; Ahrends A1; Ballingall KT4; Brooker RW5; Burke T6; Cavers S7; Dawson IK8; Elston DA9; Kerr J10; Marshall DF11; Pakeman RJ5; Trivedi C12; Wall E8; Wright F13; Ogden R14*
- Royal Botanic Garden Edinburgh, 20A Inverleith Row, Edinburgh, EH3 5LR, Scotland UK
- Scottish Natural Heritage, Great Glen House, Leachkin Road, Inverness, IV3 8NW, Scotland UK
- University of Edinburgh, Institute of Evolutionary Biology, Ashworth Laboratories, Kings Buildings, Charlotte Auerbach Road, Edinburgh, EH9 3FL, Scotland UK
- Moredun Research Institute, Pentlands Science Park, Bush Loan, Penicuik, Midlothian EH26 0PZ, Scotland UK
- James Hutton Institute, Craigiebuckler, Aberdeen, AB15 8QH, Scotland UK
- Department of Animal and Plant Sciences, University of Sheffield, Sheffield, England UK
- Centre for Ecology and Hydrology, Bush Estate, Penicuik, Midlothian, EH26 0QB, Scotland UK
- Scotland’s Rural College, Peter Wilson Building, Kings Buildings, West Mains Road, Edinburgh EH9 3JG, Scotland UK
- Biomathematics and Statistics Scotland, Peter Wilson Building, Kings Buildings, West Mains Road, Edinburgh EH9 3JG, Scotland UK
- Science and Advice for Scottish Agriculture, Roddinglaw Road, Edinburgh, EH12 9FJ, Scotland UK
- UK James Hutton Institute, Invergowrie, Dundee, DD2 5DA, Scotland UK
- Millennium Seed Bank, Royal Botanic Garden Kew, Ardingly, Haywards Heath, Sussex, RH17 6TN, England UK
- Biomathematics and Statistics Scotland, James Hutton Institute, Invergowrie, Dundee, DD2 5DA, Scotland UK
- The Royal (Dick) School of Veterinary Studies, University of Edinburgh, Easter Bush Campus, Midlothian, EH25 9RG, Scotland UK
Correspondence: [email protected]; +44 (0) 131 248 2883
* Contributed equally
By 2020, the genetic diversity of cultivated plants and farmed and domesticated animals and of wild relatives, including other socioeconomically as well as culturally valuable species, is maintained, and strategies have been developed and implemented for minimizing genetic erosion and safeguarding their genetic diversity.
Executive summary
- Aichi Target 13 (T13) focuses on the conservation of genetic diversity.
- Major challenges in implementing T13 are that the type of genetic diversity to conserve is not clearly defined, and that key issues in genetic conservation vary across different sectors (e.g., forestry vs agriculture vs other species of socio-economic importance).
- In Scotland and the UK more widely, baseline mechanisms are well established for assessing and reporting on genetic diversity in species of agricultural importance (e.g., rare livestock breeds, crop wild relatives), and a methodology has been established for ornamental plants.
- A new UK Strategy for Forest Genetics Resources was launched in 2019, creating a framework for linking forest trees into T13 reporting.
- However, there is no clear strategy to deal with ‘other species of socio-economic importance’ in Scotland, the UK or indeed elsewhere, and addressing this gap is the major focus of this report.
- There is a lack of guidance for identifying focal species of socio-economic importance, and no clear mechanism for addressing T13 for these species once they have been identified.
- To address this, we have identified a set of criteria for defining terrestrial and freshwater species of socio-economic importance in Scotland, and selected an initial list of 26 species.
- The criteria applied were:
- National conservation priority wild species.
- Species of national cultural importance.
- Species providing key ecosystem services.
- Species of importance for wild harvesting (food and medicine).
- Economically important game species.
- We then developed a simple, readily applicable scorecard method for assessing risks to the conservation of genetic diversity in these species.
- The scorecard approach is not dependent on prior genetic knowledge, and instead uses structured expert opinion assessments of whether:
- Demographic declines are likely to lead to loss of genetic diversity (genetic erosion).
- Hybridisation is likely to lead to undesirable replacement of genetic diversity.
- Restrictions to regeneration/turnover are likely to impede evolutionary change.
- For plant species where seed-banking is a viable mechanism for holding genetic resources ex situ, we also report on the representativeness of these ex situ collections.
- Overall, this scorecard provides a mechanism for incorporating ‘other species of socioeconomic importance’ into T13 actions and reporting.
- Furthermore, its application is not restricted to Aichi T13 as the approach is designed as a generic scorecard for genetic diversity. It is thus relevant to post-2020 CBD targets focusing on genetic diversity.
- Future priorities include:
- Extension to other species of socio-economic, commercial and cultural importance (with the inclusion of marine species being a particularly high priority).
- Harmonising genetic conservation strategies between sectors (drawing on commonalities), whilst minimising disruption of existing well-established methodologies within sectors.
- Greater incorporation of genomic data into monitoring genetic diversity (particularly in the agricultural and forestry sectors where data availability is potentially high).
Aims
The aim of this report is to outline a national approach for the conservation of genetic diversity suited to Scotland and applicable internationally.
Specifically – to develop a scorecard approach for wild species of cultural and socioeconomic importance as a necessary component of a national framework which also encompasses agriculture, horticulture and forestry, to promote long-term conservation of genetic diversity and address Aichi Target 13, and its subsequent post-2020 iterations.
In developing this report, we have initially restricted our focus to Terrestrial and Freshwater species. Our primary geographical focus is Scotland, set in its wider UK context.
Aichi Target 13 – definitions and current status
Definitions
Conserving genetic diversity is the focus of 2020 Aichi Target 13 (T13) of the Convention on Biological Diversity (CBD): By 2020, the genetic diversity of cultivated plants and farmed and domesticated animals and of wild relatives, including other socio-economically as well as culturally valuable species, is maintained, and strategies have been developed and implemented for minimizing genetic erosion and safeguarding their genetic diversity.
Related targets and initiatives
The conservation of genetic diversity is the explicit focus of a series of ‘State of the World’s Genetic Resources’ (SoWGR) publications:
- State of the World’s Plant Genetic Resources for Food and Agriculture.
- State of the World’s Animal Genetic Resources for Food and Agriculture.
- State of the World’s Forest Genetic Resources.
A report on State of the World’s Aquatic Genetic Resources for Food and Aquaculture is also under preparation. These SoWGR publications provide sector-specific summaries and an overview of genetic conservation issues relevant to T13.
The Global Strategy for Plant Conservation (GSPC) has been adopted by the CBD. It includes three targets that are directly relevant to T13:
- GSPC Target 5: At least 75 per cent of the most important areas for plant diversity of each ecological region protected with effective management in place for conserving plants and their genetic diversity.
- GSPC Target 8: At least 75 per cent of threatened plant species in ex situ collections, preferably in the country of origin, and at least 20 per cent available for recovery and restoration programmes.
- GSPC Target 9: 70 per cent of the genetic diversity of crops including their wild relatives and other socio-economically valuable plant species conserved.
How Aichi Target 13 has been interpreted to-date
T13 explicitly targets the genetic diversity of cultivated plants, and farmed and domesticated animals and their wild relatives. It also explicitly targets the development of genetic conservation strategies. Indicators for T13 are summarised in Text Box 1. T13 also includes “other socio-economically as well as culturally valuable species” although what this encompasses remains undefined (see Section 3d below).
Trends in genetic diversity of cultivated plants:
- Number of plant and animal genetic resources for food and agriculture secured in either medium- or long-term conservation facilities (indicator for SDG target 2.5).
- Number of plant genetic resource for food and agriculture surveyed/inventoried.
- Percentage of plant genetic resources for food and agriculture threatened out of those surveyed/inventoried.
- Number of Standard Material Transfer Agreements, as communicated to the Governing Body of the International Treaty on Plant Genetic Resources for Food and Agriculture.
Trends in genetic diversity of farmed and domesticated animals:
- Proportion of local breeds, classified as being at risk, not-at-risk or unknown level of risk of extinction (indicator for SDG target 2.5).
Trends in extinction risk and populations of wild relatives:
- Red List Index (wild relatives).
- Species Habitat Index (wild relatives).
Trends in protected area coverage of wild relatives (to be resolved):
- Species Protection Index (wild relatives)
Trends in genetic diversity of socioeconomically as well as culturally valuable species:
- No specific indicators identified.
Trends in development and implementation of strategies for minimizing genetic erosion and safeguarding genetic diversity:
- Level of implementation of global plan of actions on genetic resources for food and agriculture.
The 2016 interim report and the 2017 interim report on Aichi Target 13 from Scotland included reports on:
- Crop plants and crop wild relatives stored ex situ in seed banks.
- Crop wild relative occurrences in situ.
- Landraces stored ex situ.
- The status of rare breeds of domesticated mammals.
- The genetic status of two native species of wild deer.
At the UK level, the 2019 report to the CBD focused on effective population size for at-risk breeds of farm animals and the number of accessions of plants in germplasm collections.
In the 2014 and 2019 UK CBD reports, in the GSPC annexe, there is further reporting on coverage of ex situ collections of wild plant species. There is also reporting on the proportion of threatened horticultural cultivars that are included in conservation programmes.
Challenges and omissions for Aichi Target 13 reporting
The inclusion of agricultural genetic resources in T13 is clear cut (e.g., crop wild relatives, landraces, livestock rare breeds). However, beyond this, there is considerable ambiguity as to what could/should be reported on:
- Forest genetic resources are not explicitly listed in T13, or previously reported on by the UK, but are the subject of a global State of the World’s Forest Genetic Resources report.
- The T13 wording does not formally define the limits of ‘cultivated plants’ and hence the degree to which ornamental/horticultural genetic resources should be included remains open.
- The interpretation of “other socio-economically as well as culturally valuable species” remains open. The CBD Target 13 Technical Rationale notes this can include “selected wild species of plants and animals”, but the criteria for species selection remain unspecified. This is reflected in the absence of T13 indicators for ‘socioeconomically as well as culturally valuable species’ (Text Box 1).
The large range of different sectors and species that T13 is relevant to creates challenges in specifying appropriate metrics and indicators, and establishing success criteria. Section 4 examine these issues further.
Genetic diversity – definitions, usefulness, metrics and conservation strategies
Defining genetic diversity
Genetic diversity is a generic term for differences among individuals due to differences in their DNA sequence. For T13 it is useful to recognise that this generic definition encompasses two somewhat different components.
Genetic variability: Genetic variability relates to the presence of different genetic types, with the focus being on the number and characteristics of different genetic types.
Genetic distinctiveness: Genetic distinctiveness relates to the degree to which a given entity is different and distinct from other entities. This can include ‘evolutionary divergence’ (e.g., lineages that have been isolated for long periods of time and hence have become genetically distinct). It can also include ‘genetic purity’ (e.g. entities that have been bred to conform to a standard such as rare breeds of livestock).
Why is genetic diversity useful?
- Loss of genetic diversity can reduce fitness and elevate extinction risks of varieties, populations and species.
- Genetic diversity loss also reduces the genetic resources available to enhance species traits for human utilization.
- Genetic diversity is involved in the adaptation of populations to the environmental conditions they occur in: different populations are often genetically adapted to those local conditions (e.g. wetter vs drier places, presence of endemic pathogens).
- Genetic diversity loss can impede future adaptive responses to environmental change (e.g. to climate change or new pest and pathogens).
- Loss of genetic diversity in key individual species can have impacts on diversity in other species (e.g. genetically determined differences in the chemistry of individual trees represents a form of habitat diversity for species associated with those trees).
- Genetically distinct lineages reflect an aspect of biodiversity that may warrant conservation in their own right.
How genetic diversity problems can arise?
T13 focuses on maintaining genetic diversity and minimising genetic diversity loss.
- Genetic diversity can be lost where there is a decline in the size of a given population: all things being equal, larger populations hold more genetic diversity than smaller populations (Frankham, 1996). This principle generally holds for populations in the wild (in situ) and populations held ex situ (e.g. on a farm, in a botanic garden, in a seed bank etc.).
- Genetic diversity can be lost when parts of a species range are lost (range contractions / loss of entire populations). As genetic diversity is typically geographically structured – then loss of a species from a given area can be associated with a loss of genetic diversity associated with that area (Frankham et al., 2017).
- Where breeds or varieties are no longer maintained, the genetic characteristics of those breeds or varieties can be lost (although in many cases these genetic characteristics can be ‘re-created’ by further selective breeding).
- If hybridisation occurs between previously isolated lineages (or different species) there is the potential for a different type of genetic problem (Todesco et al., 2016). This can occur due to a straight forward loss of purity or distinctiveness (the displacement of genetic diversity of one entity by another). It can also lead to a fitness reduction in offspring and an associated population decline if there are genetic incompatibilities between the taxa that hybridise.
- Another important element for conservation of genetic diversity is the maintenance of adaptive potential and evolutionary processes. This is particularly relevant to long-lived organisms which may experience recruitment/regeneration limitations, which reduce opportunity for genetic change and adaptive evolution to new environmental conditions.
Traits of individual species can affect their sensitivity to genetic change. The ways in which traits interact with sensitivity to diversity loss are complex and generalisations are difficult. However, species which naturally inbreed (e.g. self-pollinating plant species) typically show low levels of genetic variability within individual populations (Aguilar et al., 2006). This means that loss of individuals from populations of inbreeding species has limited impacts on genetic diversity as most individuals are genetically similar. This contrasts with the situation in outcrossing species, where reductions in the size of a given population can have marked impacts on maintenance of genetic diversity.
Approaches for conserving genetic diversity
Genetic diversity can be conserved ‘in the wild’ (in situ), outside of species’ natural habitat, often in collections (ex situ), or in highly managed systems (circa situ conservation).
In situ
Conserving genetic diversity in situ has the intrinsic benefit of allowing natural evolution and genetic change as populations are exposed to new environmental conditions and pressures. A key aspect of in situ management for genetic diversity is enabling these processes to take place, and of particular importance is turnover. This is relevant for long-lived organisms (e.g., forest trees) which can persist as individuals without regeneration if management conditions are sub-optimal, thus reducing opportunities for evolutionary change. An additional basic advantage of in situ conservation is that in many cases there can be greater available land area and the ability to support more individuals (and hence more genetic diversity) than for other conservation approaches.
Ex situ
Ex situ collections (e.g. seed banks, farms, zoos, botanic gardens, plantations, arboretums, cryopreservation) provide an accessible genetic resource and a ‘safe house’ where continued survival in situ is uncertain. The approach is particularly well suited for propagules such as seeds which can be stored for long periods of time in a space-efficient fashion. The key challenges are assembly costs, storage costs, and space requirements where living individuals are the only practical ex situ option. A general challenge for ex situ collections is that removal from in situ conditions typically precludes adaptation and evolution to changes in the in situ environment. Furthermore, for ex situ living collections (e.g., zoos, botanic gardens), generational turnover can lead to adaptation to ex situ conditions which may be undesirable if reintroduction to the wild is an ultimate goal.
Highly managed systems (circa situ conservation)
Some species of agricultural importance are maintained circa situ, in highly managed systems within their native range. Examples include the management of stands of native forest trees as seed orchards, and more general management of species in an agricultural/agroforestry/urban landscape. These systems combine the benefits of the management control of ex situ collections, with some level of exposure to changes in ambient environmental conditions facilitating evolutionary change. Conversely, one limitation is that depending on the management practices applied, the species in question may be subject to atypical selection pressures leading to unwanted/unexpected evolutionary change.
The relative importance of in situ, versus circa situ, versus ex situ approaches will vary depending on the resource to conserve (Figure 1).
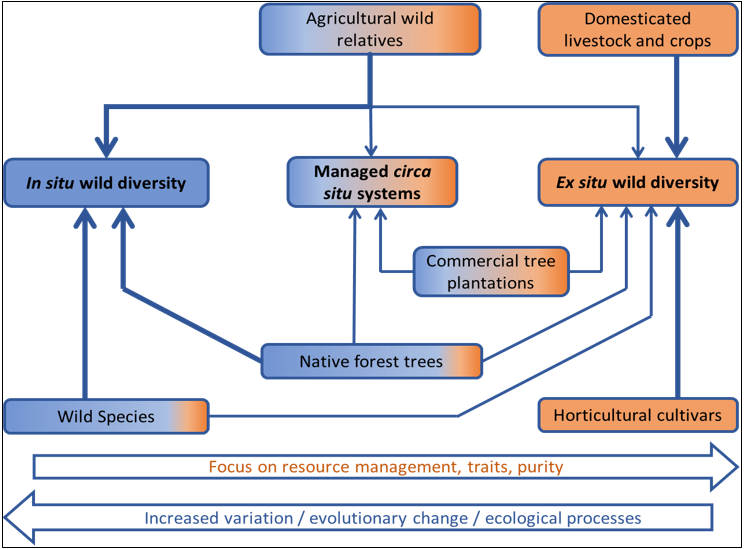
The colour shading reflects the continuum from in situ (blue) to ex situ (orange) conservation.
At one extreme, crops, livestock breeds and horticultural cultivars are highly domesticated genetic resources held almost entirely ex situ, with a strong focus on maintaining the traits and purity of these domesticated variants (Figure 1). At the other end of the spectrum are wild species, where the primary focus is on maintaining diversity and the propensity for evolutionary change (with ex situ collections representing an important ‘back-up’ resource). Wild relatives (from the same or related species) of domesticated plants and animals span the full spectrum including species which are essentially wild with potential for domestication or use in breeding programmes, through to semi-domesticated variants with a greater focus on specific traits and purity and which are incorporated into agricultural management practices of varying levels of formality (Figure 1).
The importance of in situ genetic resource conservation for wild relatives of domesticated taxa varies from country to country (some countries simply lack wild relatives of the important domesticated species). Native forest species also encompass a broad range of conditions, from wild natural populations, through to populations with greater degrees of management and utilisation. Commercial plantations of forest trees are often of exotic species, and the primary in-country mechanism for conserving genetic diversity will be tree improvement programmes and seed orchards, with existing genetic resources backed up in ex situ collections (Figure 1).
Relevant scales for consideration of genetic diversity
Geographical scale and the location of genetic resources are important for T13. Variables for consideration include the geographical source of the asset, its origin (wild, domesticated) and its conservation location (in situ in country, ex situ in country, ex situ out of country). This creates a complicated range of scenarios about where genetic conservation can be delivered, e.g.:
- Wild native germplasm in situ.
- Wild germplasm ex situ in its country of origin.
- Wild germplasm ex situ housed in a different country.
- Domesticated germplasm in the country of domestication.
- Domesticated germplasm housed in a different country.
Where genetic resources from one country are conserved in another, this raises the challenge of inter-dependencies: the resource is conserved ex situ but there may be concerns about future access to that resource by the country of origin.
A second issue relevant to geographic scale is range representation. If a species has a single occurrence in one country but is abundant in many others, then the value judgement of conserving the single occurrence is scale dependent (e.g. conserving that single occurrence is simultaneously conserving 100% of the local resource, and a trivial portion of the global resource). A key factor here is the degree to which individual countries house genetically distinct/divergent variants, and the extent to which coordination between countries can maximise complementarity.
How genetic diversity is measured/inferred
Table 1 summarises the different ways genetic diversity can be measured or inferred. There is no single measure of genetic diversity or methodology that is appropriate for all situations. As genetic diversity ultimately relates to differences in DNA sequence, an obvious starting assumption is that some measure of DNA sequence variation is the baseline unit of measurement. This, however, is not straightforward for two reasons.
- Costs and accessibility: Although DNA sequencing costs continue to fall, obtaining, managing and interpreting DNA sequence data on a large scale is still expensive and requires access to specialist skills/equipment. The launch of major biodiversity genome sequencing projects such as the Earth Biogenome Project are addressing this issue, but extensive work is required before range-wide genome sequence variation is available for most species.
- Relevance: The relationship between genetically determined traits of interest (e.g. disease resistance, adaption to a particular set of conditions) and the DNA sequence of an organism is still poorly understood outside of model species.
Conserving genetic diversity thus requires consideration of different aspects of genetic diversity (amounts/types of diversity and levels of distinctiveness); the factors which might cause genetic problems (diversity loss, unwanted genetic replacement, constraints on evolution); the relative importance of in situ, circa situ, and ex situ conservation, and the practicalities of how genetic diversity can be measured and monitored.
|
Unit of measurement/ surrogate |
Description |
Applicability |
|---|---|---|
|
Total genetically determined phenotype |
All aspects of an individual that are genetically determined (e.g. appearance, traits, adaptive characteristics) |
Extremely useful, but practically intractable in most cases. Comprehensive direct measurement of genetically determined traits requires detailed experiments as much variation among individuals is environmentally induced |
|
Selected genetically determined trait/phenotype |
Data on a selected subset of genetically determined traits or levels of fitness |
Direct measures of adaptation/fitness/traits of interest can directly inform strategies to conserve this type of diversity. This does not capture the full range of potentially important adaptive variation, and evaluation can be confounded by environmental effects unless in controlled conditions |
|
Completely sequenced genome |
Comprehensive sequence and assembly of an individual’s genome providing a full representation of its genetic make-up |
Extremely useful but still expensive to obtain for many species. Provides a way to directly compare levels of genetic variation and distinctiveness. However, the relationship between DNA sequence (even for a complete genome) and traits of interest is often still poorly understood, and presence of a complete genome may not, in itself, be informative as to e.g. the appearance or degree of adaptation to a given set of environmental conditions |
|
Genetic marker data |
DNA data from a subset of the genome gathered to assess levels of variability and distinctiveness |
Very useful measure of general amounts and distribution of genetic variation (and whether variation is higher or lower in some individuals/populations). Usually uninformative about particular traits or degree of adaptation, although marker data can be targeted to candidate genes for adaptation |
|
Ecological and environmental information |
Data on population sizes, isolation, distribution and environmental variation. Indirect measure of underlying genetic and adaptive variation |
Basic distributional data represents a surrogate measure for the distribution of genetic diversity as (a) Population size and isolation shows some correlation with genetic diversity (small/isolated populations ≈ lower diversity); and (b) Geographic distribution shows some correlation with distribution of genetic diversity (more geographically distant ≈ more different). Populations inhabiting different environments may show adaptive differences (bigger environmental differences ≈ bigger adaptive differences). Involves making assumptions/extrapolation from other studies, rather than directly measuring genetic information from the species concerned. Distributional data are widely available, data on population sizes are less widely available |
|
Collection information
|
Data on number and richness of ex situ collection events/accessions
|
Classic metric for stored material (cryopreservation, seedbanks, zoos, botanic gardens). Can provide a surrogate measure of level of coverage against an ‘ideal’ as to the degree to which variation in the wild is conserved ex situ. It is usually based on the assumptions outlined for “Ecological and environmental information” rather than directly measuring any genetic information from the species concerned |
Addressing Aichi Target 13 in Scotland/UK for agriculture, forestry and horticulture
In this section we outline the scope, current approaches, and future opportunities for genetic conservation for Agriculture, Forestry and Horticulture. These sectors have established conservation genetic strategies and reporting (e.g. Agriculture – livestock; Agriculture – plants; Horticulture), or recently developed strategies (Forestry) in the UK. For the category of ‘Other species of Socio-economic importance’, where there is no existing strategy, in Section 6 we provide much more detail and a new framework for addressing these species in the context of T13.
Agriculture – livestock
The ‘State of the World’s Animal Genetic Resources for Food and Agriculture’ is a key component of T13 and the subject of a range of interim progress reports from the UK and Scottish Governments. As well as the cultural value of traditional and rare breeds, the principal driver for the conservation of the world’s livestock genetic resources is the demand for animal derived protein to feed a human population predicted to increase to 9.7 billion by 2050 (United Nations Department of Economic and Social Affairs Population Division, 2015). This will require a significant increase in the efficiency of production over the next 30 years.
The principal terrestrial livestock species include the ruminants (cattle, sheep, goat), monogastrics (such as pig, horse) and poultry species, which are dominated by the chicken. Each of these species has a complex domestication history over the last 10,000-12,000 years and despite the domestication process and thousands of years of selection, each has maintained significant levels of genetic diversity (FAO, 2015). While goat, sheep, pig and chicken each have extant wild relatives, the wild ancestor of domestic cattle, the auroch (Bos primigenius), was driven to extinction in the early 17th century (Park et al., 2015). Aquatic species have been adapted to farming relatively recently and wild relatives are available to introduce novel diversity into farmed populations as required. For terrestrial species in the UK, over the last 300 years, hundreds of distinct breeds of each livestock species, each occupying a distinct environmental (hill, lowland) or production (meat, fibre, milk) niche were developed through traditional selective breeding (UK Country Report on Farm Animal Genetic Resources, 2012). More recently the introduction of a small number of breeds extensively selected for higher levels of productivity has replaced many of the traditional breeds leaving many under threat of extinction. Target 13 seeks the development and implementation of strategies to maintain the genetic resource represented by the traditional breeds for future resilience in the face of environmental and social change.
The current approach to the conservation (and T13 reporting) of livestock genetic diversity across the UK is through identification and monitoring of the number and effective population size of those breeds at risk. Conservation is focused through the actions and commitment of rare breed societies. This conservation strategy has been effective as no UK livestock breed has become extinct since 1973. The most recent report from the UK government on progress towards T13 suggests that the number and effective population size of most UK rare breeds are being maintained. However, this is unlikely to be true worldwide, especially in countries lacking the resources to identify and maintain traditional breeds.
The strength of the current approach is that breeds are maintained in their ‘natural’ domesticated environment where they are able to adapt over time to changes in their environment. The cost to the government is low as the rare breed societies and individual farmers take most of the breed management responsibility.
The limitations of this approach are that animals are commonly maintained in relatively small numbers, often in isolated environments where the threat of disease or statutory culling for disease control can have a devastating effect, as could farmers deciding to exit farming. High levels of genetic diversity are generally associated with a healthy breeding population which is better able to deal with changes in the environment or in disease patterns. The genetic health of rare breeds is less well defined especially in closed flocks developed from a small starting population. Maintenance of breed purity may also be an issue especially as many populations are maintained on commercial farms along with other breeds. With an aging farming population, rare breeds may be threatened by demographic and financial challenges facing farmers.
An immediate improvement to the approach would be the development of conservation strategies for each breed based on maximising and maintaining the existing genetic diversity. This would require a genome wide assessment of the diversity present and a breeding strategy tailored to the requirements of each breed allowing the exchange of genetic material between isolated populations. The draft genomes of each of the terrestrial livestock species are available and a wide range of genotyping tools have been developed to identify the genetic basis of some production traits. The identification of the genetic basis of most of the phenotypic differences between rare and highly selected breeds has not been well defined.
Agriculture – plants
The State of the World’s Plant Genetic Resources for Food and Agriculture Report gives an overview of global genetic resource challenges for crop production and food security. The report highlights the importance of crop gene pools to support human nutrition and in reducing the environmental costs of crop production. At the same time, land clearing, environmental degradation, changing agricultural practices and other pressures are resulting in a loss of plant genetic resources for the agricultural sector. The Report indicates that:
- Globally there has been progress in securing plant genetic diversity for food and agriculture in national genebanks that complement major international genebanks, such as those held by the CGIAR centres, with more than 7 million accessions held in total.
- Crop wild relatives and lesser-used but locally- or regionally-important crop species remain underrepresented in these collections, although efforts are underway to address this.
- The expansion of protected areas globally supports the in situ conservation of crop wild relatives, although there is an absence of consistently good inventory data to measure effectiveness.
- Greater coordination is required between in situ and ex situ conservation measures.
The major cereal crops important in the UK (wheat and barley, to a lesser extent oats) all originated from elsewhere but have been grown in the UK for centuries or millennia, resulting in farmer-developed landraces as well as old formally-bred varieties that are specifically adapted for UK environmental and use requirements (Schmidt et al., 2018). Apart from cereals, potatoes and oilseed rape are the main crops produced in Scotland (Scottish Government, 2018). As well as seed potatoes, Scottish farmers grow 'ware' potatoes for direct human consumption, and there are c. 21,000 hectares of vegetables and soft fruit grown in Scotland (Scottish Government, 2018).
The maintenance of UK-developed old varieties and landraces is important for future crop development, both for understanding the evolutionary trajectory and history of crop improvement and for providing a gene pool from which traits for future adaptation and production can be sourced. Cereal genetic resources in the UK are maintained mostly as ex situ seed collections, but in some case as still-cultivated resources. A good example in Scotland is landraces of bere barley still grown in Orkney, Shetland and the Hebrides
(Schmidt et al., 2018). Measures have been taken to integrate bere barley into whisky production (Martin and Chang, 2008). Similar measures could support the on-farm conservation of other crop landraces.
Since the major Scottish crops are of exotic origin, access to germplasm collections held elsewhere in the world is crucial for future production. This is currently assured through global agreements on access to resources and through collaborative multi-locational programmes of research. These initiatives may in the future be unintentionally threatened however by the implementation of access and benefit sharing arrangements under the Nagoya Protocol, which can impede germplasm exchange, and through any future political barriers to international cooperation. Since Scotland and the UK are net recipients rather than net donors from the perspective of crop genetic resource gene pools, measures to maintain and enhance access and collaboration are important.
In terms of the crop wild relatives present in the UK and Scotland, various reviews have been undertaken at UK (Maxted et al., 2007) and individual country scales (Fielder et al., 2015a; 2015b; 2016). A review for Scotland was undertaken in 2016 (Fielder et al., 2016). This identified 120 priority crop wild relative taxa. Subsequent analyses identified hotspots where many of the taxa co-occur, and where occurrences mapped to protected areas. These analyses identified areas of central and eastern Scotland that had the potential to be in situ conservation sites. These broad-brush analyses provide a foundation for subsequent more detailed planning (e.g., higher resolution spatial analyses to identify reserve boundaries and assessment of whether occurrence in protected areas is associated with optimal management for the species in question). This then provides the data required for further targeted in situ interventions for conserving genetic diversity in these species. The above reviews have also noted the relatively low representation of crop wild relatives in genebanks. The situation for Scotland is described as ‘severely lacking’ – although ongoing seed collecting efforts for the Scottish flora are adding further populations to the Millennium Seed Bank. One general challenge is the relatively large number of taxa identified as crop wild relatives and this necessitates effective prioritisation of actions. Part of this should include a review of taxonomic status of some priority taxa (e.g. Scottish small reed Calamogrostis scotica is highlighted as a priority crop wild relative species for action on account of its rarity, but its taxonomic status is highly questionable).
Target 13 reporting for UK and Scottish plant genetic resources relevant to agriculture has focused on the number of accessions held in genebanks and recorded in the EURISCO catalogue. No formal reporting has yet been undertaken on the status of crop wild relatives in the wild in the UK or Scotland.
Forestry
Global challenges for the conservation of Forest Genetic Resources are summarised in the
State of the World’s Forest Genetic Resources, and at a European level by the Pan European strategy for genetic conservation of forest trees (de Vries et al., 2015). At a global scale there are c. 80,000-100,000 species of trees, with estimates of about 34,000 tree species of socioeconomic importance being used on a daily or weekly basis by people around the world (FAO, 2014). Landuse changes, illegal logging, forest degradation and deforestation represent major global threats to forests and forest genetic resources. In the UK there are > 40 common native tree and shrub species, with birch, oak, and ash being the most abundant broadleaved species (Forestry Commission, 2018). There are just three native conifer species (Scots pine, yew, and juniper), but conifer plantations of Sitka spruce,
Scots pine and larches form a major component of UK forest cover (Forestry Commission, 2018). In Scotland/UK, climate change and emerging pest and pathogens are major imminent sources of concern for tree and forest health.
The goals for the conservation of forest genetic resources are well established and include:
For native trees to ensure:
- That a broad range of adaptive variation for important species is conserved (in situ and ex situ), as many tree species show marked adaptation to different environments.
- That natural regeneration is occurring at a sufficient number of sites to enable adaptation to environmental change and emerging threats.
- That when existing populations are augmented with planted material to overcome regeneration limitations, the planted material is suitably adapted and genetically variable (and that there is an appropriate supply of seeds to enable this).
For commercial species to ensure:
- That unnecessary loss of genetic diversity is avoided during tree improvement programmes and when producing seed in seed orchards.
A current major approach to genetic conservation in European forest trees is to identify the range of climatic zones within which either native species are present or commercially important exotic species are naturalised. Within each of these zones the aspiration is to designate at least one dynamic gene conservation unit for each target species. These are forest areas which contain sufficient numbers of individuals to retain high genetic diversity (ideally 500, but as low as 50 where only scattered individuals are available) and that receive gene flow from other sites. Dynamic gene conservation units are managed to encourage natural regeneration and the action of natural selection such that ongoing adaptation to changing environmental conditions, including anthropogenic changes, occurs. A network of 3,200 dynamic conservation units have been established across Europe, encompassing 4,000 different populations or about 400 species, although 80% of these represent just five economically important species.
While the scientific underpinning for achieving genetic conservation using dynamic gene conservation units has been developed in detail, and practical guidelines for implementation have been published, no such units were designated in Scotland and the wider UK before
2019. In 2019, a Strategy for UK Forest Genetic Resources was launched (Trivedi et al., 2019). One aim outlined in this strategy is to use the UK’s existing network of protected sites as a starting point to establish a formally recognised set of Gene Conservation Units for UK tree species. When this step is addressed, this will align and integrate forest genetic conservation in the UK, with the wider European forest genetic resource landscape. This process has started, with the first Gene Conservation Unit in the UK being designated in 2019 at Beinn Eighe in Scotland.
Substantial efforts have been made to secure extant native forest genetic resources in the UK in ex situ or circa situ collections. The UK National Tree Seed Project is working to seedbank representative material from across the UK to obtain seed collections from 75 tree species, and as of November 2019, this had resulted in the collection of more than 10 million seeds from over 10,600 trees across the UK. Work is also underway to develop cryopreservation approaches for species whose seeds are not possible to store in conventional seedbanks. In addition, extensive forest germplasm is held by various tree breeding programmes and in seed orchards.
A challenge for genetic resource conservation in tree breeding programmes and seed orchards is to maintain genetic diversity. There can be a major trade-off between the desirability of a uniform planting stock vs. maintaining sufficient genetic variability to promote long-term viability, and selective breeding for particular traits can result in an overall narrowing of genetic variation. Likewise, serious loss of genetic diversity is possible in tree breeding programmes where production populations are based on seed from orchards containing a limited number of parents, and where vegetative propagation of individuals from small numbers of half- and full-sib families is employed to produce planting stock.
Various genetic and genomic programmes on tree species have been undertaken, and genome sequences are available or being generated for species such as ash, dwarf birch, apple, sweet cherry, poplar, aspen, shrub willow, oak and Norway spruce (Chen et al., 2018). Such a rich set of genomic resources makes the design of efficient genetic assays for UK forest tree species relatively tractable and affordable. This would facilitate the monitoring of change in genetic diversity, allowing problems to be highlighted, and remedial measures to be applied.
To-date there has been no specific reporting against T13 for forest genetic resources for Scotland/UK. One element of T13 is … “strategies have been developed and implemented for minimizing genetic erosion and safeguarding their genetic diversity”. The Strategy for UK Forest Genetic Resources itself represents a contribution to T13, and the number and geographical coverage of dynamic gene conservation units can be included in reporting as they become established. In addition, reporting on the representation of native forest tree species in seedbanks/ex situ collections is a simple targeted measure for this sector.
Ornamental plants/horticultural genetic resources
General strategies for the conservation of horticultural genetic resources (in the sense of ornamental plants) are not well developed, and there is no State of the World genetic resource plan for ornamentals. Key genetic issues include (a) maintaining the diversity in wild relatives of horticulturally important species, and (b) maintaining the range of individual cultivars. The situation is analogous to that of livestock.
The overview method that is being used for tracking the genetic resources of cultivated plants in the UK involves adopting an IUCN red-listing approach to assess whether cultivars are threatened (Seymour, 2012). This is undertaken via the Plant Heritage Threatened Plants Project. The obvious strength of this is that it uses a well-established methodology for threat assessment adapted for horticultural collections. This approach has been included in the UK CBD reports via Target 9 of the Global Strategy for Plant Conservation. One obvious challenge is the very large diversity of cultivars used as ornamental plants (resulting in a substantial work load). A related issue with regards to allocation of effort is that of ‘recreatability’. Many cultivars could be recreated by further selective breeding, and hence the protection of diversity in wild progenitor species is arguably more efficient, although, as with livestock breeds, there can be a strong cultural connection to the conservation of some domesticated variants. As the primary source of cultivars in the UK horticultural sector is from outside of the UK, there is a strong focus in Scotland/UK on ex situ collections of these cultivars (many of which are held by amateur enthusiasts), as opposed to conservation of wild in situ diversity.
Addressing Aichi Target 13 in other species of social/economic importance and cultural value
The category of ‘other species’ has no over-arching strategic framework for implementation and no suggested indicators. The sheer breadth of this category means that the range of genetic issues that are relevant will encompass those of the other sectors outlined above (and indeed species composition will not necessarily be mutually exclusive). To address T13 for this category we have adopted two key steps. Firstly, we have established a process for defining and selecting ‘other species of socio-economic importance and cultural value’. Secondly, we have developed a T13 genetic scorecard which is flexible enough to cope with many different issues and types of species, whilst having sufficient coherence to enable comparative reporting.
Defining species for inclusion
There is no agreed national list of species of socio-economic and/or cultural value for Scotland. Generating such a list involves making subjective decisions. Cultural valuation is subjective and dependent on the stakeholder group being asked and the way the question is asked. Likewise, the outcomes of economic valuations are methodology dependent, and even where an agreed method is available, robust valuations are simply not available for many species.
Given these challenges, we have adopted a pragmatic approach to selecting a subset of species for initial focus. We identified a set of categories reflecting the reasons why a species might be considered socio-economically or culturally important:
- Species prioritised for conservation value.
- Species identified as being culturally important.
- Species providing important ecosystem services.
- Game species (wild species of direct commercial value through hunting).
- Species collected for food or medicine (forage species).
Under each category we then looked for existing approaches for prioritising species. Where an existing approach was available, we used it, and the top species were included in our list.
Where no clear approach was available, we outlined a simple method for selecting species. Our aim was not to provide comprehensive coverage of species, nor to identify a set of indicator species. Rather it was to develop criteria for identifying priority species and then to assess their genetic status.
We chose 26 species as being a manageable number to consider in the first instance. We selected a similar number of species from each of the above categories. We also aimed to include representatives of different taxonomic lineages, although overall there is strong representation of vertebrates and vascular plants.
Finally, we checked whether our selected species would be appropriate targets for assessing genetic diversity conservation. Some species may be socio-economically/culturally valuable but relatively uninformative in the context of monitoring changes in diversity over time. For example, species of critical conservation concern where population numbers are low and genetic diversity has already been lost, may not represent appropriate species for monitoring future changes.
Using the above general rationale, our approach for selecting species, by category, was as follows:
- Species of conservation value: We considered a species to be a priority if it was a previously identified target for conservation action. To select Scottish species, we worked from the Species Action Framework (SAF). Species were included in the SAF list based on their conservation need combined with opportunities for delivering conservation benefits in Scotland. We selected five species from the SAF list, aiming to give broad taxonomic coverage (two invertebrates, one vertebrate, one fungus and one vascular plant).
- Species of cultural value: We selected species of cultural value, primarily using the results of a public questionnaire to identify culturally important species in Scotland. The definition used in the questionnaire of important species was: “important for any reasons including for conservation reasons, for their own personal enjoyment, as economically important (e.g. fishing), simply their favourites, as symbols of Scottish identity or just that they are nice to see.” This provided relatively long lists of animals and plants “important to the Scottish population”; the top ten of each is shown in Table 2. We selected species from this informal list, including representative terrestrial or freshwater plant and animal taxa. Finally, we added one more species of cultural importance, the European ash, Fraxinus excelsior. Although not on the top ten list produced in 2006, it is a species of high cultural interest (Rackham, 2014) and with a recent surge in attention associated with catastrophic decline due to the ashdieback pathogen.
|
Rank |
Top ten animals |
Top ten plants |
|---|---|---|
|
1 |
Red deer or roe deer |
Heather |
|
2 |
Red squirrel |
Scots pine |
|
3 |
Golden eagle |
Bluebell/Harebell |
|
4 |
Dolphin, porpoise or whale |
Oak |
|
5 |
Wild salmon |
Thistle |
|
6 |
Badger |
Rowan |
|
7 |
Osprey |
Scottish primrose |
|
8 |
Otter |
Poppy |
|
9 |
Butterfly |
Ferns |
|
10 |
Robin |
Orchid |
- Species providing key ecosystem services: In the absence of a prioritised list of key providers of ecosystem services for Scotland, we adopted a highly simplified approach and included the top three (non-planted) vascular plant species by cover in Scotland based on the Countryside Survey 2007 (Norton et al., 2009). The rationale for this approach is that high cover levels can reasonably be taken as indicative of high standing biomass and productivity.
Using our land-cover approach, there was one case where this did not lead to a species level selection. ‘Total bryophyte cover’ was third on the Countryside Survey 2007 for ground cover. Reflecting the importance of bryophyte communities for carbon storage, we selected Sphagnum papillosum as the UK’s most important moss species relevant to this ecosystem service.
To extend the phylogenetic coverage beyond plants, we included another exemplar taxon. We selected the common frog, Rana temporaria, as the most widely distributed vertebrate in mainland Scotland and an important regulator of invertebrate populations. In total, five species were included to represent ecosystem services: common frog, S. papillosum and three vascular plant species. An obvious further extension would be additional species playing key roles in ecosystem processes (e.g. earthworms and their importance for soil processes) or in specific regulating services (e.g. tree species and flood mitigation).
- Species of importance for food and medicines: Whilst foraging for food and traditional medicines are not as important to people in a post-industrial nation like Scotland, it is still of cultural value. We included this category in recognition of its greater importance in countries with less disturbed ecosystems and a greater reliance on wild food sources. Information on the level of use of forage species came from a survey of foragers. We included four species that were among those with highest frequency of reported use.
- Important game species: Economic data were available for assessing the value of some game species (PAEC, 2015). We based our selection on a combination of this economic data and taxon type, selecting the top fish (Atlantic salmon and brown trout), mammal (roe and red deer) and bird (red grouse) species.
Our finalised set of 26 'other socio-economically as well as culturally valuable species' for Scotland is summarised in Table 3, along with the categories they were selected under. Several species were directly selected under multiple categories (e.g. Atlantic salmon, heather and red deer were identified as culturally important species, as well as being identified by other categories), and many other species have attributes/uses relevant to multiple categories.
|
- |
Selection criteria Conservation
|
Selection criteria Culturally Important |
Selection criteria Ecosystem Services |
Selection criteria Food/medicines |
Selection criteria Game |
Taxonomic group |
|---|---|---|---|---|---|---|
|
Papillose bog-moss Sphagnum papillosum |
- |
- |
yes |
- |
- |
Bryophyte |
|
Scots pine Pinus sylvestris |
- |
yes |
- |
- |
- |
Vascular plant |
|
Raspberry Rubus idaeus |
- |
- |
- |
yes |
- |
Vascular plant |
|
Oak Quercus spp. |
- |
yes |
- |
- |
- |
Vascular plant |
|
Woolly willow Salix lanata |
yes |
- |
- |
- |
- |
Vascular plant |
|
Heather Calluna vulgaris |
- |
Yes |
Yes |
- |
|
- |
|
Blaeberry Vaccinium myrtillus |
- |
- |
- |
yes |
- |
Vascular plant |
|
British bluebell Hyacinthoides non-scripta |
- |
yes |
- |
- |
- |
Vascular plant |
|
Harebell/Scottish bluebell Campanula rotundifolia |
- |
yes |
- |
- |
- |
Vascular plant |
|
European ash Fraxinus excelsior |
- |
yes |
- |
- |
- |
Vascular plant |
|
Elderberry Sambucus nigra |
- |
- |
- |
yes |
- |
Vascular plant |
|
Yorkshire fog Holcus lanatus |
- |
- |
yes |
- |
- |
Vascular plant |
|
Purple moor-grass Molinia caerulea |
- |
- |
yes |
- |
- |
Vascular plant |
|
Hazel gloves Hypocreopsis rhododendri |
yes |
- |
- |
- |
- |
Fungus |
|
Chanterelle Cantharellus cibarius |
|
- |
- |
yes |
- |
Fungus |
|
Freshwater pearl mussel Margaritifera margaritifera |
yes |
- |
- |
- |
- |
Mollusc |
|
Great yellow bumblebee Bombus distinguendus |
yes |
- |
- |
- |
- |
Insect |
|
Sea trout/brown trout Salmo trutta |
- |
- |
- |
- |
yes |
Fish |
|
Atlantic salmon Salmo salar |
- |
yes |
- |
- |
yes |
Fish |
|
Common frog Rana temporaria |
- |
|
yes |
- |
- |
Amphibian |
|
Golden eagle Aquila chrysaetos |
- |
yes |
- |
- |
- |
Bird |
|
Red grouse Lagopus lagopus |
- |
|
- |
- |
yes |
Bird |
|
Red squirrel Sciurus vulgaris |
- |
yes |
- |
- |
- |
Mammal |
|
Scottish wildcat Felis silvestris |
yes |
- |
- |
- |
- |
Mammal |
|
Red deer Cervus elaphus |
- |
yes |
- |
- |
yes |
Mammal |
|
Roe deer Capreolus capreolus |
- |
- |
|
- |
yes |
Mammal |
Genetic assessment process
We developed a genetic scorecard based on a central working principle of a need to be flexible to allow use not only in Scotland but in other countries. Factors that vary among countries include:
- The presence of endemic divergent genetic lineages: Countries and regions which have not experienced major perturbations such as extinctions due to glacial cycles are more likely to have accumulated endemic divergent phylogenetic lineages, than countries with ‘young’ biotas which have been largely assembled over the last 10,000 years following ice-sheet retreat. A focus on divergent lineages is likely to be more appropriate in regions with long term historical climatic stability, compared to e.g. recent glaciated boreal countries like Scotland.
- Resource availability: The capacity of a given country to report on genetic diversity in wild species will depend on the resources available, the level of knowledge of a given biota, and the diversity and scale of the country in question. Key requirements of an effective system for reporting on wild species genetic diversity are scalability and applicability in situations where resource availability is limited.
- Geographical scales of data holdings: Where spatial data are used for reporting, a practical issue relates to the scale at which data are held. Additional steps may be required to up-scale or down-scale reporting where different datasets are held and curated at different levels (e.g. state vs national level). This is a particular issue for the UK where considering priority species for Scotland requires either steps to extract Scotland-specific data from UK-wide compilations, or consideration of issues at a UK scale for Scottish priority species.
Our process involves the following stages (further details in Table 4):
- Summary of relevant genetic conservation issues for the species in question, to articulate key issues and relevant species traits that may impact on susceptibility to genetic problems.
- A statement of international importance of national genetic diversity for the species, to contextualise the assessment.
- Evaluation of key genetic risks facing in situ populations:
- Diversity loss – focusing on population declines (e.g. general diversity loss), the loss of functional diversity (e.g. important traits or adaptive differences), or loss of populations that are likely to hold unique evolutionary lineages.
- Hybridisation – focusing on risks of elevated mixing of genetic diversity leading to negative consequences (e.g. genetic pollution, genetic swamping, outbreeding depression).
- Low turnover – an approximation of the degree to which turnover is restricted. This is particularly important for long-lived species, such as forest trees, where lack of regeneration can impede evolutionary change.
- Overall in situ threat assessment based on expert opinion leading to a ‘low-mediumhigh’ genetic risk categorisation based on species attributes and the risks faced by the species.
- A statement of confidence on the overall in situ threat assessment. This step aims to transparently capture the level of certainty among experts as to the risk categorisation.
- The level of representation in ex situ collections. This step was undertaken for plant species which are particularly well suited to holding ex situ collections in seed banks.
The analysis was undertaken at the UK level and we assessed:
- The number of 10 km squares represented in seedbank collections, as a proportion of the 10 km squares in which the species is known to occur.
- The extent of occurrence (EOO) of seed bank collections as a proportion of the EOO of in situ populations (where EOO = the minimum convex polygon around all points).
- The Great Britain (GB) seed zones represented in seedbank collections, as a proportion of the GB seed zones in which the species occurs.
- A summary of existing management actions relevant to the species in Scotland.
- A final combined overall ‘traffic light score’ on how well T13 is addressed for the species. This is an expert opinion based on the species, its risks and current management responses.
|
Header |
Information required |
|---|---|
|
Context Background |
Overview of what is known about genetic diversity in the species and key relevant biological traits (e.g. breeding system, mode of reproduction, any previous genetic studies). |
|
Context Current threats |
Summary of threats facing the species (e.g. grazing, overharvesting, predation, persecution, climate change etc.). |
|
Context Contribution of Scottish population to total species diversity |
Assessment of the relative contribution of taxon in Scotland to total species diversity (e.g. whether Scotland has internationally important range representation, endemic subspecies, Scottish population differentiation). |
|
Genetic risks Diversity loss: population declines
|
Narrative assessment of likelihood of diversity loss (e.g. small or reduced population sizes, range contraction or fragmentation). Incorporation of direct genetic evidence if available. |
|
Genetic risks
Diversity loss: functional variation
|
Narrative assessment of likelihood of important functional diversity (e.g. particular threats to specific ecotypes or phenotypic trait classes). Incorporation of direct genetic evidence if available. |
|
Genetic risks
Diversity loss: divergent lineages |
Narrative assessment of likelihood of loss to divergent populations (e.g. known or suspected distinct lineages). This can be based on morphological, geographical or historical data, or if available direct genetic evidence of divergent lineages. In animals, but less commonly in plants, such lineages are often referred to as evolutionary significant units. |
|
Genetic risks
Hybridisation/ introgression |
Narrative assessment of likelihood of loss of genetic integrity via inter-specific hybridisation or inappropriate mixing. This can be based on direct genetic data, morphological assessments of hybridity, or proximity and abundance of known threats. |
|
Genetic risks
Low turnover/ constraints on adaptive opportunities |
Narrative assessment of reproduction and recruitment as an indicator of potential for evolvability. If there are limitations to recruitment, the narrative should indicate the proportion of populations that are affected. |
|
Cumulative risk summary In situ genetic threat level |
Expert opinion classifying in situ genetic risk as serious, moderate, or negligible. |
|
Cumulative risk summary Confidence in situ threat level |
Confidence based on evidence quality and agreement. |
|
Cumulative risk summary Ex situ representation |
Narrative and/or quantitative summary of representation in viable and accessible ex situ conservation resource (e.g. number of seed zones represented in ex situ collection, range representation of seed zones in ex situ collection). |
|
Cumulative risk summary Current conservation actions |
Narrative and ‘check-box’ summary of current approaches/in situ interventions to mitigate threat. |
|
Cumulative risk summary Overall T13 status |
Summary of genetic conservation status, encompassing risk and efficacy of current mitigating actions. |
|
Cumulative risk summary Overall T13 status explanation |
Succinct explanation/justification of overall status. |
|
Assessor |
Name of person or group who undertook the assessment. |
|
Reviewer |
Name of person or group who reviewed the assessment. |
Quantification of levels of genetic risk
Compared to metrics focusing on demographic changes (e.g. IUCN Red Listing), there is no universally accepted standard for measuring genetic diversity, and by extension no universal standard for classifying loss of genetic diversity into different threat categories. Different genetic diversity metrics measure different properties (e.g. diversity of allelic variants within a population, diversity of allelic variants within individuals, overall differences between populations, the nature of the genetic differences between populations). Furthermore, different ways of measuring genetic diversity result in different sensitivities to genetic diversity loss (e.g. a population could simultaneously lose half of its genetic variation for some regions of its genome and lose no variation for others).
A second difference between genetic change and demographic change relates to the reversibility of losses. Individuals and populations experience demographic decline due to mortality that can be replenished by local births or migration. Genetic variation can be replenished by migration, but not where unique genetic variants are lost – mutation rates operate on a temporal scale that is often measured in thousands of years. Thus, all things being equal, in perfect conditions for a species, a loss of 50% of its genetic diversity would have a much longer recovery time than loss of 50% of its individuals.
Collectively, these differences preclude direct adoption/translation of threat categories from standard IUCN Red-Listing to genetic variation. Instead, we have developed generalised statements to steer expert opinion to a relatively consistent classification of genetic problems. A deliberate aim is not to constrain definitions too tightly, in recognition that different data types and scenarios may frequently be experienced. Using 2010 as a baseline reference point, the scorecard we have adopted assesses contemporary genetic issues, and likely future issues, during a 25-year window from the point of assessment.
Genetic risks are classed as:
- Negligible: No obviously detectable genetic problems occurring or expected over the next 25 years.
- Moderate: Moderate genetic problems occurring or expected over the next 25 years; e.g.:
- Moderate loss of populations which are likely to contain unique diversity (e.g. resulting in losses of up to 25% of important genetic types/distinct populations).
- Clearly observable fitness problems in up to 25% of populations due to low genetic variation and subsequent inbreeding depression.
- Marked and clearly observable loss of genetic integrity by hybridisation for up to 25% of populations.
- Severe restrictions on regeneration/recruitment/reproduction in many or most populations of long-lived species limiting evolutionary change in the immediate future.
- Serious: Serious genetic problems occurring or expected over the next 25 years; e.g.: - Severe loss of populations which are likely to contain unique diversity (e.g. resulting in losses of > 25% of important genetic types/distinct populations).
- Loss of any highly divergent endemic lineages that are globally unique.
- Strong clearly observable fitness problems in >25% of populations due to low genetic variation and subsequent inbreeding depression.
- Marked and clearly observable loss of genetic integrity by hybridisation at >25% of populations.
For quantification of certainty we use the approach adopted for the UK Biodiversity Climate Change Impacts Report Card (Figure 2). This combines the level of agreement among experts and the type of available evidence. The overall confidence level is governed by the lowest score for either expert agreement or evidence availability.
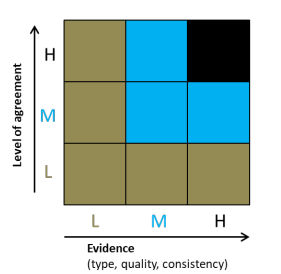
For quantification of certainty we use the approach adopted for the UK Biodiversity Climate Change Impacts Report Card (Figure 2). This combines the level of agreement among experts and the type of available evidence. The overall confidence level is governed by the lowest score for either expert agreement or evidence availability.
Worked example of a genetic scorecard for Pinus sylvestris
A worked example genetic scorecard is shown in Table 5 (Scots pine, Pinus sylvestris). Completed scorecards for 26 species are presented in the Supplementary Report to Scotland’s Biodiversity Progress to 2020 Aichi Targets Report 2019.

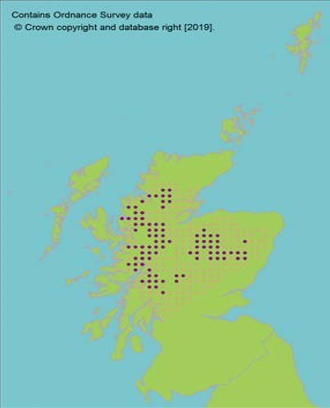
Map showing Scots pine across Scotland. Natural stands represent only 10% of trees in Scotland. Genetic marker studies show large amounts of neutral genetic diversity. Some evidence of adaptive differentiation in Scotland from west to east (Salmela, 2011; Donnelly et al., 2018).
|
- |
|---|---|
|
Context Background |
Hermaphrodite, wind pollinated, widely distributed tree. Present in 84 natural stands, often small and fragmented (dark circles on map, light circles are plantations). Natural stands represent only 10% of trees in Scotland. Genetic marker studies show large amounts of neutral genetic diversity. Some evidence of adaptive differentiation in Scotland from west to east (Salmela, 2011; Donnelly et al., 2018). |
|
Context Current threats |
Plant pathogens represent the major emerging threat (Dothistroma septosporum races introduced on Corsican and lodgepole pine) (Piotrowska et al., 2018). |
|
Context Contribution of Scottish population to total species diversity |
Molecular evidence for putative separate lineage in north western Scotland, although nuclear markers indicate very low differentiation, even from continental Europe (Ennos et al., 1997). Scotland does, however, contain a uniquely oceanic adapted population (Ennos et al., 1997; Donnelly et al., 2018). |
|
Genetic risks Diversity loss: population declines
|
Multiple small populations with no regeneration coupled with a biased age-structure towards older trees compromises the sustainability of many populations. However, there is limited risk of imminent genetic diversity loss due to high levels of standing variation in adult trees (assuming no catastrophic population losses due to pathogens). |
|
Genetic risks Diversity loss: functional variation
|
The general persistence of the species across its range in Scotland is not threatened, which minimises likely loss of adaptive variation. There are risks to loss of high elevation populations across its range which may lead to some loss of adaptive variation. |
|
Genetic risks Diversity loss: divergent lineages |
Limited divergence from European populations precludes loss of major divergent lineages. The most genetically distinct populations are in the north west of Scotland around Shieldaig. These populations are not currently threatened. |
|
Genetic risks Hybridisation/ introgression |
Buffer zones in which planting of non-local seed is prohibited around existing native stands limit risk to loss of integrity from exotic stands. |
|---|---|
|
Genetic risks Low turnover/ constraints on adaptive opportunities |
Deer grazing is a major limitation on turnover and regeneration, but the risk is mitigated in c. 20% of populations where active management is in place. |
|
Cumulative risk summary In situ genetic threat level |
Moderate (in the face of emerging pathogen threats, major limitations to regeneration present a moderate risk of genetic variation loss and constraints to adaptation). |
|
Cumulative risk summary Confidence in in situ threat level |
High (assessment based on good demographic data and direct data on genetic variation, population differentiation and biology). |
|
Cumulative risk summary Ex situ representation |
Seeds from 13 10km squares held at the Millennium Seed bank, including all 5 UK ‘standard’ tree seed zones in which native stands occur, with 68% ex situ coverage of its wild extent of occurrence. |
|
Cumulative risk summary Current conservation actions |
Grazing controls at c. 20% of sites promote regeneration providing adaptive opportunities. Establishment of Gene Conservation Unit at Beinn Eighe National Nature Reserve safeguards some variation. |
|
Cumulative risk summary Overall T13 status |
Moderate risk; Mitigation effective |
|
Cumulative risk summary Overall T13 status explanation |
Despite the fragmented nature and small size of many populations, longevity of individual trees minimises imminent loss of genetic diversity. Management to promote regeneration supports some ongoing evolutionary processes, and wide representation of all seed zones in seed banks likely catches main adaptive variation. |
|
Assessor |
Richard Ennos, University of Edinburgh |
|
Reviewer |
Stephen Cavers, Centre for Ecology and Hydrology Peter Hollingsworth, Royal Botanic Garden Edinburgh |
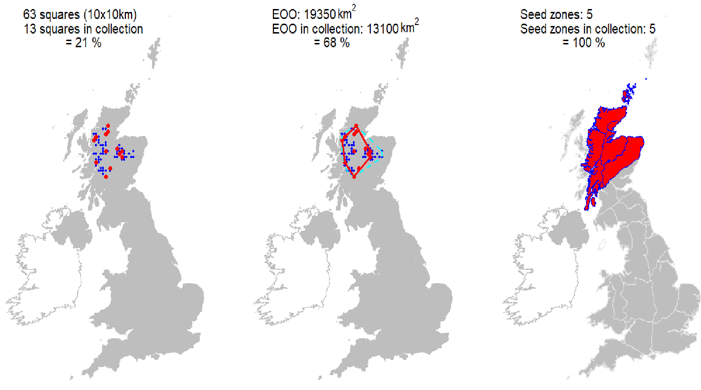
Seeds from 13 10km squares held at the Millennium Seed bank, including all 5 UK ‘standard’ tree seed zones in which native stands occur, with 68% ex situ coverage of its wild extent of occurrence.
FAQs on the adopted approach for ‘other species of socioeconomic/cultural importance’
Why not have a greater focus on generating DNA data to make the monitoring more comparable and repeatable?
- An important aim of the approach outlined above is to enable relatively rapid progress and evaluation of the key issues to consider, regardless of the level of existing genetic data on the species concerned.
- Although the accessibility of population genetic and population genomic data is growing rapidly, it will simply not be available for many important species for the foreseeable future.
- Even where direct genetic data are available, conclusions/recommendations typically still draw very heavily on demographic and environmental information due to the difficulty in obtaining information on adaptive variation or fitness from most DNA-based studies.
- Thus, the approach is designed to accommodate DNA data, where available, but not to make it a pre-requisite to progress in the immediate future.
Do the selected species act as indicators for wider conservation of genetic diversity or is the genetic approach developed here targeted to focal species?
- The species are not selected as indicators. The approach developed here is based on focal species which are of sufficient importance to warrant consideration/tracking in their own right.
- Different species are likely to respond differently to environmental/anthropogenic stressors and management interventions. However, it should be possible to carefully select some focal species to act as indicators for others. This is most likely to work for co-occurring species with similar management requirements and life history characteristics.
What is the rationale for the focus on just three issues (genetic diversity loss, hybridisation and recruitment limitations) for assessing in situ genetic health?
- Although drivers of genetic diversity change are complex and multi-faceted, at their core they can be distilled down into these three issues, namely (i) whether genetic diversity is being lost, (ii) whether it is being replaced, or (iii) whether restrictions on evolutionary processes impede ongoing and future genetic adaptation.
- The most wide-ranging of these is genetic diversity loss (point i). This can have multiple causes (e.g. reduced population sizes, population losses, natural or artificial selection). However, these all ultimately boil down to a reduction in the number of individuals contributing to subsequent generations compared to background levels. Such reductions can be approximated in broad terms by demographic observations and/or expert opinion.
- Gene flow within species (intra-specific hybridisation) and gene flow between species (inter-specific hybridisation) are natural ecological processes. However, there are some cases where elevated gene flow can lead to genetic replacement/genetic swamping (point ii). The most obvious examples are elevated threats to genetic integrity due to intra or inter-specific hybridisation with related invasive non-native species or non-native commercial cultivars/stock. Observations on morphological appearance can give an approximation as to the extent of the issue, even where direct genetic data are not available.
- Constraints on recruitment hampering evolutionary processes (point iii) are particularly applicable to tree species. Management practices (e.g. grazing regimes) can reduce levels of seedling recruitment. This can in turn reduce opportunities for gradual natural selection to allow a population to track environmental change. This can be viewed as a temporal loss of adaptive genetic variation. The extent of this issue can be assessed in very general terms by the degree to which populations of the focal species show signs of reproduction followed by recruitment.
What information sources were used to develop the general guidelines?
- The general working principles used here are well established based on population genetic theory and many empirical studies (Frankham et al., 2017).
- A summary of the evidence base of human impacts on genetic variation (Mimura et al., 2017), which in turn draws on multiple previous meta-analyses.
- A Biodiversity Impacts report card technical paper (Implications of climate change for genetic diversity and evolvability in the UK (Neaves et al., 2015)) also provides a recent summary of evidence and agreement for general principles governing the circumstances under which genetic diversity is likely to be lost and its consequences.
How long does it take to complete each species assessment?
- The completion time for each species has varied from one hour where the assessors had direct expertise and detailed knowledge on the species concerned, to one day where no species-specific knowledge was available to the assessors.
Given the heavy reliance on expert opinion, what are the controls against subjective decisions impacting on the outcome?
- The approach was selected to identify broad-brush categories of risk to minimise subjective decisions resulting in different categorisation.
- Panel members were selected from a wide variety of academic backgrounds and included practitioners to reduce taxonomic biases and specialist ‘tunnel vision’.
- Uncertainty is explicitly quantified, and the assessment was checked by a minimum of two reviewers. For the species rigorously tested so far, there was close agreement of the characterisation of the genetic conservation status.
How might the approach be extended?
- More formal standardised integration of direct genetic data (e.g. via the use of a standardised set of data) would enable directly comparative monitoring of genetic diversity in different species, should the resources become available.
- More formal use of distributional data and modelling approaches would improve quantification of where demographic changes are likely to be occurring and hence where genetic diversity loss may occur (e.g. high-resolution distributional data, trend data, and/or species distribution modelling to estimate occurrences for data-deficient species).
- Greater incorporation of quantitative environmental variables such as assessment of the environmental range in which a species occurs, and the degree to which populations from across that range are represented in ex situ holdings, would increase the robustness of surrogate assessments of the representativeness of ex situ collections. Going beyond seedbanks and living plant collections for quantifying the extent of ex situ holdings (e.g. cryobanks) will become increasingly important as the number of species and number of individuals conserved in these fashions continues to grow.
Summary and concluding remarks
The approach proposed here for reporting on genetic diversity in wild species of socioeconomic and/or cultural importance fills a major gap in the process for addressing Aichi Target 13. Coupled with existing approaches for Agriculture and Horticulture, and the recent development of a UK Forest Genetic Resources strategy, all key sectors can now be covered in genetic diversity assessments for Aichi Target 13 and future derivative targets.
The approaches to genetic conservation clearly vary between these sectors, reflecting sector needs and the relative importance of in situ, circa situ, and ex situ methods (summarised informally in Table 6). In Scotland, and the UK more generally, for commercial forestry, livestock, horticulture and crop/crop relatives there is strong importance of circa situ and/or ex situ collections, reflecting both the importance of the managed environment for these sectors and the lower importance of Scotland/UK wild populations as a source of useful traits (compared to populations elsewhere). In contrast, for native forest tree species, and wildspecies of socio-economic and cultural importance, there is a proportionately greater importance and emphasis on in situ resources in Scotland/UK, reflecting their primary location/use in natural and semi-natural systems. However, despite this generalisation, there is considerable overlap in the general principles of genetic conservation between sectors. Table 7 summarises key next steps and priorities for genetic conservation among sectors. For in situ conservation, the general framework of assessing constraints on evolvability, diversity loss and genetic swamping can be applied to all sectors. Likewise, formalised assessments of the representativeness of ex situ holdings could be applied in a common framework across different sectors. However, the sector-specific standard approaches are widely used, and any changes (e.g. livestock or crop wild relative reporting) run the risk of unnecessary disruption of established practices.
|
Sector |
In situ Global |
In situ Scotland |
Circa situ Global |
Circa situ Scotland |
Ex situ Global |
Ex situ Scotland |
|---|---|---|---|---|---|---|
|
Agriculture – livestock breeds |
1 |
1 |
2 |
1 |
3 |
3 |
|
Agriculture – livestock wild relatives |
3 |
1 |
3 |
1 |
2 |
2 |
|
Agriculture – landraces |
1 |
1 |
3 |
3 |
2 |
2 |
|
Agriculture – crop wild relatives |
3 |
2 |
1 |
1 |
3 |
3 |
|
Forestry – native species |
3 |
3 |
2 |
2 |
2 |
2 |
|
Forestry – commercial plantations |
1 |
1 |
3 |
3 |
3 |
3 |
|
Horticulture – cultivar diversity |
1 |
1 |
1 |
1 |
3 |
3 |
|
Horticulture – wild relatives |
3 |
2 |
1 |
1 |
3 |
3 |
|
Wild species of socioeconomic/cultural importance |
3 |
3 |
2 |
2 |
2 |
2 |
|
Sector |
Key next steps |
|---|---|
|
Agriculture – livestock |
|
|
Agriculture – plants |
|
|
Forestry |
|
|
Horticulture |
• Undertake further assessments of the presence of threatened cultivars in National Plant Collections or other active conservation programmes. |
|
Wild species of socioeconomic/ cultural importance |
|
References
Aguilar, R., Ashworth, L., Galetto, L. & Aizen, M. A. 2006. Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecology Letters, 9, 968-980.
Chen, F., Dong, W., Zhang, J., Guo, X., Chen, J., Wang, Z., Lin, Z., Tang, H. & Zhang, L. 2018. The sequenced angiosperm genomes and genome databases. Frontiers in Plant Sciences, 9, doi.org/10.3389/fpls.2018.00418.
De Vries, S., Alan, M., Bozzano, M., Burianek, V., Collin, E., Cottrell, J., Ivankovic, M., Kelleher, C., Koskela, J., Rotach, P., Vietto, L. & Yrjänä, L. 2015. Pan-European strategy for genetic conservation of forest trees and establishment of a core network of dynamic conservation units. Biodiversity International.
Donnelly, K., Cavers, S., Cottrell, J.E. & Ennos, R.A. 2018. Cryptic genetic variation and adaptation to waterlogging in Caledonian Scots pine, Pinus sylvestris L. Ecology and Evolution, 8, 8665-8675.
Ennos, R.A., Sinclair, W.T. & Perks, M.T. 1997. Genetic insights into the evolution of Scots pine, Pinus sylvestris L., in Scotland. Botanical Journal of Scotland, 49, 257-265.
FAO, 2015. The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture. In: Scherf, B.D. & Pilling, D. eds. FAO Commission on Genetic Resources for Food and Agriculture Assessments. Rome.
Fielder, H., Brotherton, P., Hosking, J., Hopkins, J.J., Ford-Lloyd, B. & Maxted, N. 2015a. Enhancing the conservation of crop wild relatives in England. PLOS ONE, 10, e0130804.
Fielder, H., Burrows, C., Woodman, J., Ford-Lloyd, B.V. & Maxted, N. 2015b. Enhancing the conservation of crop wild relatives in Wales. New Journal of Botany, 5, 178-191.
Fielder, H., Smith, C., Ford-Lloyd, B. & Maxted, N. 2016. Enhancing the conservation of crop wild relatives in Scotland. Journal for Nature Conservation, 29, 51-61.
Frankham, R. 1996. Relationship of population size to genetic variation in wildlife. Conservation Biology, 10, 1500-1508.
Frankham, R., Ballou, J.D., Ralls, K., Eldridge, M., Dudash, M.R., Fenster, C.B., Lacy, R.C.
& Sunnucks, P. 2017. Genetic management of fragmented animal and plant populations. Oxford, Oxford University Press.
Martin, P. & Chang, X. 2008. Bere whisky - rediscovering the spirit of an old barley. Brewer & Distiller International, 2008, 41-43.
Maxted, N., Scholten, M., Codd, R. & Ford-Lloyd, B. 2007. Creation and use of a national inventory of crop wild relatives. Biological Conservation, 140, 142-159.
Mimura, M., Yahara, T., Faith, D.P., Vázquez-Domínguez, E., Colautti, R.I., Araki, H.,
Javadi, F., Núñez-Farfán, J., Mori, A.S., Zhou, S., Hollingsworth, P.M., Neaves, L.E., Fukano, Y., Smith, G.F., Sato, Y.-I., Tachida, H. & Hendry, A.P. 2017. Understanding and monitoring the consequences of human impacts on intraspecific variation. Evolutionary Applications, 10, 121-139.
Neaves, L.E., Whitlock, R., Piertney, S.B., Burke, T., Butlin, R.K. & Hollingsworth, P.M.
2015. Implications of climate change for genetic diversity and evolvability in the UK. Living With Environmental Change Biodiversity Climate Change impacts report card technical paper 15.
Norton, L.R., Murphy, J., Reynolds, B., Marks, S. & Mackey, E. C. 2009. Countryside survey: Scotland results from 2007. Centre for Ecology & Hydrology, Scottish Government, Scottish Natural Heritage.
PAEC, 2015. The benefits and volume and value of country sports tourism in Scotland.
Park, S.D.E., Magee, D.A., Mcgettigan, P.A., Teasdale, M.D., Edwards, C.J., Lohan, A.J.,
Murphy, A., Braud, M., Donoghue, M.T., Liu, Y., Chamberlain, A.T., Rue-Albrecht, K.,
Schroeder, S., Spillane, C., Tai, S., Bradley, D.G., Sonstegard, T.S., Loftus, B.J. & Machugh, D.E. 2015. Genome sequencing of the extinct Eurasian wild aurochs, Bos primigenius, illuminates the phylogeography and evolution of cattle. Genome Biology, 16, 234.
Piotrowska, M.J., Riddell, C., Hoebe, P.N. & Ennos, R.A. 2018. Planting exotic relatives has increased the threat posed by Dothistroma septosporum to the Caledonian pine populations of Scotland. Evolutionary Applications, 11, 350-363.
Rackham, O. 2014. The Ash Tree. A Little Toller Monograph. Dorset, Little Toller Books.
Salmela, M. 2011. Adaptive genetic variation in Scots pine (Pinus sylvestris) in Scotland. PhD, University of Edinburgh.
Schmidt, S.B., George, T.S., Brown, L.K., Booth, A., Wishart, J., Hedley, P.E., Martin, P., Russell, J. & Husted, S. 2018. Ancient barley landraces adapted to marginal soils demonstrate exceptional tolerance to manganese limitation. Annals of Botany, 123, 831-843.
Scottish Government, 2018. Agriculture Facts and Figures for 2017.
Seymour, K. 2012. Conserving cultivars. The Plantsman, September 2012, 154-159.
Todesco, M., Pascual, M.A., Owens, G.L., Ostevik, K.L., Moyers, B.T., Hübner, S., Heredia, S.M., Hahn, M.A., Caseys, C., Bock, D.G. & Rieseberg, L.H. 2016. Hybridization and extinction. Evolutionary Applications, 9, 892-908.
Trivedi, C., Cavers, S., Atkinson, N., Clark, J. & Cottrell, J. 2019. A Strategy for UK Forest
Genetic Resources, Royal Botanic Gardens, Kew.
United Nations Department of Economic and Social Affairs Population Division, 2015. World Population Prospects: The 2015 Revision. New York.

Disclaimer: Scottish Natural Heritage (SNH) has changed its name to NatureScot as of the 24th August 2020.
At the time of publishing, this document may still refer to Scottish Natural Heritage (SNH) and include the original branding. It may also contain broken links to the old domain.
If you have any issues accessing this document please contact us via our feedback form.






