NatureScot Research Report 1283 - Disturbance Distances Review: An updated literature review of disturbance distances of selected bird species
Year of publication: 2022
Authors: Goodship, N.M. and Furness, R.W. (MacArthur Green)
Cite as: Goodship, N.M. and Furness, R.W. (MacArthur Green) Disturbance Distances Review: An updated literature review of disturbance distances of selected bird species. NatureScot Research Report 1283.
Keywords
Human disturbance; bird behaviour; Flight Initiation Distance; Alert Distance; Minimum Approach Distance; Buffer zones.
Background
Since 2007, Scottish Natural Heritage (now NatureScot) have referred to bird disturbance distance information presented in Ruddock and Whitfield (2007) to provide advice and guidance relating to casework involving human disturbance and protected bird species present in Scotland. However, since the 2007 publication, new disturbance response information in relation to human activity has become available. The aim of the current report is to update disturbance distances for species presented in Ruddock and Whitfield (2007) as well as to provide disturbance distance information for a range of additional protected bird species that regularly feature in Environmental Impact Assessments (EIAs) but were not included in Ruddock and Whitfield (2007).
NatureScot commissioned MacArthur Green to undertake a literature review to identify distances at which disturbance could be caused by human related activities to a number of protected UK bird species present in Scotland during the breeding and nonbreeding seasons. All potential sources of human disturbance referenced in the literature were included in the review. Bird disturbance distances were recorded in a wide range of environments including inland sites (e.g. uplands, lowlands, inland waterbodies and streams), coastline (e.g. shoreline, intertidal areas and nearshore waters) as well as offshore areas (including islands and offshore waters). The literature was searched for disturbance distances that were measured in terms of Alert Distance (AD), Flight Initiation Distance (FID) and Minimum Approach Distance (MAD), and for qualitative evidence on bird disturbance. The disturbance distances were collated into a Bird Disturbance Response (BDR) database for 65 bird species that were selected by NatureScot. This report provides an account for each species summarising: quantitative information available in terms of AD/FID and MAD, recommended protection buffer distances, the likely sensitivity of each species to human disturbance activities and the quality of information available.
Main findings
- Wild bird disturbance distances caused by a wide range of human related activities are presented for a total of 65 bird species.
- Recommended buffer zones are provided for each species.
- A total of 23 out of 65 protected bird species were assessed as having a high or a medium to high sensitivity to disturbance from human related activities. EIAs in relation to human activity and development will require greatest consideration to potential disturbance impacts for these species with high sensitivity to disturbance, and to apply appropriate mitigation in areas where these species are likely to be present.
- A total of 31 out of 65 species were assessed as having a medium sensitivity to disturbance from human related activities. This means that these species may tolerate some disturbance caused by human related activities, but the extent of disturbance caused to individual birds could depend on a wide range of factors including levels of habituation to disturbance.
- Few species (11 out of 65) were considered to have a low or a low to medium sensitivity to human disturbance. It is important to note that all bird species assessed in this review (including high, medium and low sensitivity species) are likely to vary in their response to human related disturbance in different areas depending on habituation to disturbance and other factors. Therefore, each assessment for future EIAs needs to be on a site-specific basis, taking account where possible of local circumstances that may influence bird sensitivity.
- A number of data gaps in the bird disturbance distance database are identified in this report and recommendations are provided for future research to fill these gaps.
Acknowledgements
We would like to thank NatureScot, especially Jen Graham, Dr Andy Douse and Andrew Stevenson for the clear guidance on interim drafts of this report. We also thank Dr Larry Griffin for his personal observations included for common scoter and Simon Cohen for his observations included for purple sandpiper.
Abbreviations
Alert Distance (AD)
Bird Disturbance Response (BDR)
Environmental Impact Assessment (EIA)
Flight Initiation Distance (FID)
Intergovernmental Panel on Climate Change (IPCC)
Special Protection Area (SPA)
Introduction
Scottish Natural Heritage (hereafter referred to by its operating name ‘NatureScot’) commissioned MacArthur Green to undertake a literature review to provide a list of disturbance distances caused by human related activities for a selected range of protected bird species. This report updates disturbance distance information presented in Ruddock and Whitfield (2007) which has underpinned NatureScot advice and guidance relating to disturbance. Since 2007, new disturbance response information in relation to human activity has become available for a range of protected bird species present in Scotland; the latest data (published up to summer 2021) are included in the current report. In addition, the current report includes a range of additional protected bird species that regularly feature in Environmental Impact Assessments (EIAs) but were not covered in Ruddock and Whitfield (2007).
This report follows a similar format to the NatureScot research report 1096 that provided information on the effects of disturbance caused by seaweed hand-harvesting on protected marine and coastal bird species (Goodship and Furness, 2019). Similar to the 2019 report, the current review first created a Bird Disturbance Response (BDR) database providing distances at which disturbance to birds could be caused by human related activities. For each species, the current review summarises disturbance distances in the BDR database and makes suggestions for buffer zones; the overall sensitivity of each species to human disturbance is estimated and the level of confidence in these conclusions within a Scottish context is provided. Knowledge gaps identified during the review process are also presented in this report. Recommendations for potential future monitoring programmes and research are provided with a focus on filling these gaps.
Potential impact pathways causing bird disturbance
A wide range of human activity including recreational pursuits and commercial activity may disturb protected bird species (for examples of types of human disturbance, see The Bird Disturbance Response database section
In the UK, some form of human disturbance occurs in most environments where wild bird species are present during the breeding and nonbreeding seasons. These environments include: inland sites (including uplands, lowlands, inland waterbodies and streams), coastal sites (including the shoreline, intertidal areas and nearshore waters) as well as offshore areas (including islands and offshore waters).
The impact of a human disturbance event (e.g. a pedestrian walking across a moorland, a motorboat out at sea, etc) may directly affect bird behaviour (e.g. disrupting foraging activity while the bird alarm calls, or forcing the bird to fly away from the source of disturbance, etc). This change in behaviour brought about by the disturbance event may mean that birds are disturbed from their initial activity and/or are displaced from their initial chosen location. The effect of disturbance and displacement on birds may change their energy intake/expenditure, alter their breeding success and ultimately impact their survival; some of these changes include, but are not limited to, the following:
- Changes to breeding location, timing of breeding, breeding strategy and success;
- Changes to foraging location, time spent foraging, food source, energy intake and daily energy budgets;
- Changes to roosting location and time spent at rest; and
- Changes to migration routes, stop-over locations and seasonal energy expenditure.
In addition, human disturbance may also indirectly affect bird behaviour through habitat alteration (for example habitat loss though development or agricultural practices) and/or alteration of predator numbers.
Habituation and other factors influencing disturbance distance
This review provides a guide to indicate which species are likely to be disturbed by human activities. However, it is important to keep in mind that a great many factors influence disturbance responses of birds. Even species that are considered to have a low sensitivity to human disturbance (see Assessing sensitivity to disturbance section) may be disturbed in some areas at certain times of the year and more sensitive species will also vary in their disturbance response depending upon the specific situation at the time of the disturbance event. Therefore, each study assessing bird disturbance needs to be on a site-specific basis, taking into account the context.
It is important to note that all bird species assessed in this review are, to some degree, likely to habituate to disturbance and are therefore likely to vary in their response to human disturbance in different areas. If birds are present in a highly disturbed area, then it is likely that these birds will show a high degree of habituation to disturbance and tolerate a shorter disturbance distance (Keller, 1989; Baudains and Lloyd, 2007; Ellenberg et al., 2009; Ross et al., 2015; Vincze et al., 2016). Similarly, if a site is secluded where there is little general disturbance, then birds are more likely to react to human presence at a greater distance (e.g. Bötsch et al. 2018; Samia et al. 2017). Habituation may be prevented in some locations depending on other factors, such as where birds are exposed to shooting. For example, goosanders Mergus merganser can become habituated to people in protected locations such as Hogganfield Loch Local Nature Reserve in Glasgow, where they will feed on grain and bread provided by people and will come within a few metres of people there, and on the River Kelvin, Glasgow, where they will tolerate people walking past them within a few tens of metres (Bob Furness, pers. obs.). In contrast, goosanders on salmon rivers where there has been sustained shooting of goosanders to protect fish stocks, such as the Tweed, will immediately fly away when a person appears over 100m away (Bob Furness, pers. obs.).
The distance at which a bird moves away from a source of human disturbance is often quantified as a Flight Initiation Distance (FID) and this can be understood in terms of a behavioural response involving a trade-off between avoidance of predation risk and acquiring sufficient resources, such as food. Climatic variation is one of the many factors that influence responses to disturbance (Díaz et al. 2021); one important factor relevant in Scotland appears to be the effect of cold weather/starvation affecting the behaviour of shorebirds and waterfowl in winter. It is well understood that these birds allow people to approach much more closely under extreme cold weather conditions, because the trade-off between predation risk (represented by an approaching person) and starvation risk (caused by freezing weather preventing foraging) has been altered by extreme cold weather conditions. It should therefore be noted that birds may in adverse conditions be less able to show the ‘luxury’ of alert behaviour or flight initiation in response to disturbance, although, paradoxically, the impact of disturbance under such severe conditions may be greatly increased. Díaz et al. (2021) showed that FIDs of a sample of 229 bird species decreased with increasing temperature and rainfall, which they interpret as demonstrating that FID responds to foraging success (the assumption being that for the bird species studied the foraging success declines with increasing temperature and rainfall). They also found that FIDs were influenced by urbanisation, by latitude, and by bird body mass. Urbanisation has also been shown to strongly reduce FIDs of birds in other studies (e.g. Carlen et al., 2021; Charutha et al., 2021; Nyatanga et al., 2021).
Other factors that may influence disturbance responses of birds include, but are not limited to the following: predation risk, FIDs being shorter in locations with fewer predators (Díaz et al., 2021), bird population trend (Díaz et al., 2021), what the source of disturbance is (Lethlean et al., 2017); species of the focal bird in the study (Blumstein, 2006); individual character of the focal bird, flock size and species construction in which the focal bird is present (Mori et al., 2001); the size of the focal bird (Blumstein et al., 2004; Mikula et al., 2018; Díaz et al., 2021), behaviour of the focal bird at the time it is disturbed (Liley et al., 2011; Liley and Fearnley, 2012; Lilleyman et al., 2016), energetic requirements of the focal bird (Gill et al., 2001; Beale and Monaghan, 2004), seasonal constraints (Mikula et al., 2018), whether the source of disturbance is visual or acoustic or both and whether the source of disturbance is novel to the focal bird (McLeod et al., 2013), disease status of the focal bird (Møller, 2008a), exposure of the birds to hunting pressures (Madsen, 1998a,b; Gnanapragasam et al., 2021); to mention just a few.
Weston et al. (2021) compared FIDs of African and Australian birds. Controlling for phylogeny, they found smaller FIDs among African species than Australian species when comparing residents, but not migrants. They concluded that resident African birds are more tolerant of humans, perhaps in relation to the history of cohabitation between humans and birds.
In addition, it should be recognised that birds learn to respond in an appropriate way to perceived risks from human activities. For example, whooper swans Cygnus cygnus at Hogganfield Loch accept food from people, but recognise that a bird ringer carrying a pole with a hook represents a threat worth avoiding and remain further away under those circumstances (Bernie Zonfrillo, pers. comm.). Eider ducks Somateria mollissima, learn the sound of the engine of the powerboat used to chase them away from mussel farms, and move away in anticipation of being chased when they hear the approaching engine noise underwater, but ignore other underwater noises (Ross, 2000). The subtle changes in behaviour of birds as a consequence of learning will alter responses to human disturbance of local populations with specific histories of interacting with people.
Definition of disturbance response (AD/FID)
There are three ways disturbance responses are typically measured, as defined below. As part of the literature review process, evidence of these three responses for each species was collated, where it was available.
AD: Alert Distance (AD) is defined as the distance at which a bird or group of birds starts to show alert behaviour (e.g. head up, alarm calling, staring at the source of disturbance, aggressive display, chicks startled, crouching or flattening on the nest etc) rather than sleeping, foraging or preening behaviour when approached by a disturbance agent (such as a person, or powerboat) (Livezey et al., 2016).
FID: Flight Initiation Distance (FID) is defined as the distance at which a bird or group of birds starts to escape (by walking away, running away, swimming away, taking flight, or diving) when approached by a disturbance agent (such as a person, or powerboat). This distance is assumed to reflect the trade-off between costs of escape (energetic costs of flight plus loss of food intake during the period of disturbance) and the risk associated with staying put (inferred predation risk) (Mikula et al., 2018).
MAD: Minimum Approach Distance (MAD) is defined as the minimum distance at which humans should be separated from wildlife to avoid any disturbance to the behaviour of the wildlife (Livezey et al., 2016). This distance should be such that the wildlife does not show an alert response to the presence of human activity and does not show flight initiation. Estimates of MAD can therefore be informed by measurement of AD and/or FID. MAD is commonly referred to as a buffer distance which can be determined by management, based on evidence from observed behaviour of birds.
Buffer zone: Buffer zone is defined in this report as a range of buffer distances that can be used to protect birds from human disturbance.
Although the above definitions are convenient for quantification of bird responses to human disturbance, it should be recognised that bird heart rate may be increased by exposure to human disturbance before alert behaviour or flight initiation responses are evident. Increased heart rate and increased levels of stress hormones have physiological costs and so disturbance may have subtle impacts even on birds that are not clearly showing behavioural responses to disturbance.
Buffer Zones
We were asked by NatureScot to recommend buffer zones for each study species and have done so. However, we emphasise that whereas AD and FID measurements are empirical data collected using agreed scientific methods, estimates of buffer zones must be based on policy decisions. Those should, of course, be evidence-based, but need also to consider a wide range of other aspects such as site-specific context, conservation status and importance of the focal population, and other pressures and threats affecting the population. Therefore, the estimates of buffer zones we suggest should be seen as indicative and not fixed limits that would be appropriate in all situations.
It is considered beyond the scope of this report to provide buffer zones for individual disturbance activities. For the majority of species the data isn't available to support such conclusions for the following reasons:
1) There often isn't enough data in a consistent format for any one activity type in a season to be able to confidently state a buffer range;
2) For species which do have a relatively large number of AD/FID records, disturbance distances within a species recorded in different studies can vary widely for a large number of reasons. It may often be the case that the source of activity isn't always the main factor determining the distance at which a bird responds to disturbance;
3) Following from this, there can be a large overlap in the range of disturbance distances recorded for different activities, this makes it very difficult to set a meaningful buffer zone for individual activities;
Due to the reasons listed above, providing individual buffer zones for different activities wasn't possible, however an attempt has been made to suggest a generalised buffer for the breeding season and/or non-breeding season for each species.
For species where it is possible to do so (e.g. Mallard), some text has been added to the species section to say what the highest FID/AD was recorded for different types of activity.
Bird species potentially affected by human disturbance
The 65 bird species that are the focus of this report are those which NatureScot identified could potentially be disturbed by humans on breeding and/or nonbreeding grounds in Scotland and give rise to conservation concerns as a result. The full list of species is presented in Table 1. These species are designated under the Birds Directive (EC Directive on the conservation of wild birds 2009/147) Article 4.1, listed in Annex 1 as being rare or vulnerable, as well as those birds listed under Article 4.2 as being regularly occurring migratory species. These bird species are afforded protection within Natura 2000 sites (including Special Protection Areas (SPAs). All wild bird species in the UK are also protected under the Wildlife and Countryside Act (W&CA) (1981), as amended by the Nature Conservation (Scotland) Act 2004. Some sensitive species are listed on Schedule 1 of the Act and receive enhanced protection against disturbance during the breeding season. Birds listed under Schedule 1A of the Act may not be intentionally or recklessly harassed at any time in the year (e.g. including at roost sites) and the nests of birds listed under Schedule A1 of the Act are protected all though the year, even when not in use (SNH, 2014).
The scientific name along with the common name of each focal species is listed in Table 1; these names are also repeated at the start of each species account. Protected bird groups which may potentially be disturbed by human activities and which are covered in this report include: swans and geese (family Anatidae), ducks (family Anatidae), grouse (family Tetraonidae), divers and grebes (families Gaviidae and Podicipedidae), diurnal raptors (families Accipitridae and Falconidae), waders (families Charadriidae,Haematopodidae, Phalaropidae and Scolopacidae), terns (family Sternidae), owls (family Strigidae and Tytonidae) and some other species (families Caprimulgidae, Coraciiformes, Fringillidae, Paridae and Rallidae). These family groups include both breeding and nonbreeding UK species.
Data gaps
This review has identified that, for some species, there is a lack of quantitative information available on AD and FID values. Some of these species with missing quantitative disturbance distance data have been assessed to have a medium or high sensitivity to disturbance through non-quantitative studies. The species listed below have one or fewer AD/FID records from human disturbance in the BDR database. These species therefore represent a data gap for studies (see Recommendations for further research section) investigating the impacts of human activity on bird disturbance:
- White-fronted goose, Anser albifrons (one FID pedestrian record);
- Bean goose, Anser fabalis (one FID pedestrian record);
- Greater scaup, Aythya marila (no AD/FID records);
- Common scoter, Melanitta nigra (no AD/FID pedestrian records);
- Slavonian grebe, Podiceps auratus (no AD/FID pedestrian records during the breeding season);
- White-tailed eagle, Haliaeetus albicilla (no AD/FID pedestrian records);
- Red kite, Milvus (no AD/FID pedestrian records);
- Marsh harrier, Circus aeruginosus (one FID pedestrian record);
- Hen harrier, Circus cyaneus (no AD/FID pedestrian records);
- Honey buzzard, Pernis apivorus (one FID pedestrian record);
- Hobby, Falco subbuteo (no AD/FID pedestrian records);
- Peregrine falcon, Falco peregrinus (no AD/FID pedestrian records);
- Merlin, Falco columbarius (one FID pedestrian record);
- Purple sandpiper, Calidris maritima (no AD/FID records);
- Red-necked phalarope, Phalaropus lobatus (no AD/FID records);
- Little tern, Sternula albifrons (no AD/FID records);
- Sandwich tern, Thalasseus sandvicensis (no AD/FID records);
- Arctic tern, Sterna paradisaea (one FID pedestrian record);
- Short-eared owl, Asio flammeus (no AD/FID pedestrian records);
- Tawny owl, Strix aluco (one FID pedestrian record);
- Barn owl, Tyto alba (no AD/FID pedestrian records);
- Corncrake, Crex (one FID pedestrian record); and
- Nightjar, Caprimulgus europaeus (one FID pedestrian record).
Study aims
The aim of this study was to collate AD and FID responses of a range of protected bird species to human disturbance, relative to recreation and other activities in Scotland. The outputs of this project will be used by NatureScot to provide advice and guidance to inform decisions on applications relating to disturbance.
The key objective was to carry out a thorough review of literature relating to disturbance responses of the species listed in Table 1 and compile the information into a database. The current report provides a compilation of species accounts which summarise the information held within the database. We encourage the updating of the database as further data become available.
Methods
The Bird Disturbance Response database
A summary of how the BDR database was constructed is provided below, for a full description, please see NatureScot Research Report 1096 (Goodship and Furness, 2019).
A literature search for information on quantitative disturbance response distances measured worldwide in terms of ADs, FIDs and MADs of focal UK protected bird species was extracted from academic scientific publications as well as ‘grey literature’ reports monitoring disturbance distances. Data were obtained not only from Scottish/UK studies but also from other European and worldwide studies (including those taking place in North America, Australia, Asia and Africa) that had been translated into English.
Studies recording AD/FID and MAD distances during the breeding and nonbreeding season that were included in the BDR database included the following sources of human disturbance:
Sources of human disturbance
- Recreational pedestrian disturbance (e.g. walking, running, cycling, climbing, horse riding, bait digging, egg collecting and hunting);
- Recreational use of nearshore waters (e.g. both motorised and non-motorised watercraft including kayak, jet skis, motorboats, yachts);
- Working vessels (e.g. commercial ferries, fishing vessels, tankers, cruise ships, offshore wind-farm vessels);
- Animal disturbance (e.g. cattle and dogs);
- Agricultural disturbance (e.g. tractors and 4x4 vehicles); and
- Aircraft and drone disturbance.
The BDR database quantitative studies are summarised for each species in the species accounts (see Results – Species accounts section).
Twenty-four (mostly non-UK) species were included in the BDR database as “stand-in species” to supply additional quantitative data for 16 UK species with little available quantitative data. Stand-in species belong to the same family and have similar ecologies compared with their UK counterparts; the following species were included:
Stand in species
- Tundra swan, Cygnus columbianus (standing in for whooper swan);
- Tule greater white-fronted goose, Anser albifrons elgasi (standing in for Greenland white-fronted goose);
- Brent goose, Branta bernicla (standing in for barnacle goose);
- Australasian shoveler, Anas rhynchotis (standing in for Northern shoveler);
- Pochard, Aythya farina and tufted duck, Aythya fuligula (standing in for scaup);
- Great crested grebe, Podiceps cristatus (standing in for Slavonian grebe);
- Bald eagle, Haliaeetus leucocephalus and African fish eagle, Haliaeetus vocifer (standing in for white-tailed eagle);
- Black kite, Milvus migrans (standing in for red kite);
- African marsh harrier, Circus ranivorus (standing in for marsh harrier);
- Rough-legged buzzard, Buteo lagopus (standing in for common buzzard);
- Lesser kestrel, Falco naumanni (standing in for kestrel);
- Prairie falcon, Falco mexicanus (standing in for peregrine falcon);
- Least tern, Sterna antillarum (standing in for little tern);
- Barred owl, Strix variata (standing in for tawny owl);
- Azure kingfisher, Ceyx azureus and Malachite kingfisher, Alcedo cristata (standing in for European kingfisher);
- Willow tit, Parus montanus; marsh tit, Parus palustris; blue tit, Parus caeruleus; coal tit, Periparus ater and great tit, Parus major (standing in for crested tit); and
- Parrot crossbill, Loxia pytyopsittacus (standing in for common crossbill and Scottish crossbill).
Due to small available data sample size and close ecological similarity, two species, common crossbill Loxia curvirostra and Scottish crossbill L. scotica, were considered together in one account.
In addition to quantitative studies, non-quantitative studies are provided in each species account of this report, primarily to help with assessing sensitivity to disturbance where quantitative data were limited.
Assessing sensitivity to disturbance
The sensitivity of each species to human disturbance was in part assessed through the maximum AD/FID record held within the BDR database as follows:
Sensitivity category
- Maximum recorded AD/FID value > 500m = High sensitivity.
- Maximum recorded AD/FID value between 500 and 50m = Medium sensitivity.
- Maximum recorded AD/FID value <50m = Low sensitivity.
However, in addition to the maximum recorded AD/FID value, non-quantitative information on disturbance response was also used to assess likely sensitivity to disturbance. Non-quantitative information was especially used in the assessment of species where there was limited quantitative data evidence and low agreement between references. Using a combination of quantitative and non-quantitative information, the overall likely sensitivity of each species to human disturbance was evaluated. Species for which quantitative data were scarce tended to be species with low sensitivity to human disturbance, as published studies have tended to focus on the species of high sensitivity.
Assessing the quality of disturbance response distances
The quality of the quantitative AD/FID records held within the BDR database was assessed in terms of “level of evidence” and “degree of agreement” between references in order to determine the level of confidence that should be placed in the conclusions of these studies within a Scottish context (Mastrandrea et al., 2010). For each species, a chart (Figure 1) constructed by the Intergovernmental Panel on Climate Change (IPCC; Mastrandrea et al., 2010) was used to assess level of evidence and degree of agreement. The principle of the IPCC chart when applied to the current review is that the quality of the quantitative information is most robust when there are multiple, consistent independent lines of high-quality evidence.
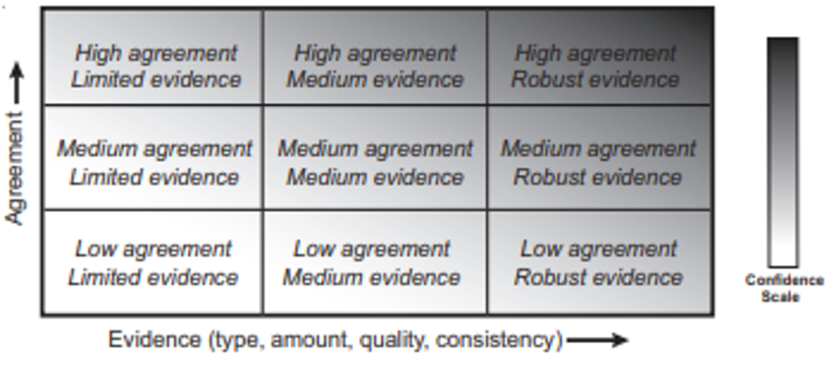
The matrix has evidence (type, amount, quality and consistency) along the x axis and agreement along the y axis. Confidence increases towards the top-right corner as suggested by the increasing strength of shading where evidence is robust and agreement high. Generally, evidence is most robust when there are multiple, consistent independent lines of high-quality
The level of evidence was categorised in terms of “robust”, “medium” or “limited” and was evaluated by combining the total number of AD and FID records (one record = one AD/FID value for each source of disturbance in each reference) during the breeding and nonbreeding seasons, together with the number of named sources of human disturbance (e.g. pedestrian, motorised watercraft, aircraft etc.) as follows:
Level of evidence category
- ≥15 AD/FID records with ≥4 disturbance sources = Robust evidence.
- ≥15 AD/FID records with <4 disturbance sources = Medium evidence.
- 5 to 14 AD/FID records with ≥2 disturbance sources = Medium evidence.
- 5 to 14 AD/FID records with 1 disturbance source = Limited evidence.
- ≤4 AD/FID records with ≤4 disturbance source = Limited evidence.
The degree of agreement between AD/FID records for each species both within the same reference and also between different references was evaluated; the breeding season and nonbreeding seasons were assessed separately. The degree of agreement was categorised in terms of “high” (i.e. AD/FID values were very similar within/between references), “medium” (i.e. there was agreement between some references, other references were dissimilar) or “low” (i.e. little agreement in AD/FID values within/between references).
Assessing buffer zone ranges
The buffer zones suggested in this report to protect each presented bird species from human disturbance during the breeding and nonbreeding seasons are intended as a guide only.
For some species, published studies have previously recommended buffer zones; where these buffer zones are available, they have been incorporated into the suggested buffer range presented in this report. Buffer zones have also been estimated, where possible, from quantitative studies that have recorded AD/FID and MAD distances during the breeding and nonbreeding seasons. For species which lack quantitative data, buffers have been estimated from non-quantitative studies. For species which lack data for one season, or where buffers are considered to be similar between both seasons, a single buffer has been provided to include both breeding and nonbreeding seasons.
A precautionary approach has been used in the estimation of buffer zones in this report; the distance at which birds of the same species respond to disturbance often overlap between different disturbance sources, therefore general buffer zone ranges are presented for the breeding and nonbreeding seasons, rather than specific buffers for different sources of disturbance.
Species accounts – table content
For each species, a table summarising the AD/FID as well as MAD/buffer zones contained within the BDR database is presented. Each table summarises the sensitivity of the species in question to human disturbance, states the quality of quantitative AD/FID records held within the BDR database and provides a suggested buffer zone range to protect the species from human disturbance during the breeding and nonbreeding seasons. Each table contains the following headings and content:
Conservation status
- UK legislation under the Wildlife and Countryside Act 1981, listed in Schedule 1 for birds afforded special protection (Scottish Government, n.d.);
- UK conservation status under Birds of Conservation Concern 5 (BoCC5; Stanbury et al., 2021);
- European legislation under the Birds Directive (European Commission Directive on the conservation of wild birds (2009/147) Article 4.1, listed in Annex 1 as being rare or vulnerable) (European Commission, 2010); and
- European conservation status under the International Union for the Conservation of Nature (IUCN) European Red List of Birds (BirdLife International, 2021a).
UK status
- UK Breeding/wintering/migration status in British Trust for Ornithology (BTO) BirdFacts (BTO, n.d.); and
- Scottish status was also added to this section if different from UK status (Forrester et al., 2012).
UK and Scottish population estimate
- Breeding and wintering numbers of birds in the UK (Woodward et al., 2020);
- Breeding and wintering numbers of birds in Scotland (Forrester et al., 2012); and
- Breeding population of raptors in Scotland/UK (Challis et al., 2020).
UK long-term trend
- UK distribution and trends: BTO Bird Atlas 2007-11 (Balmer et al., 2013);
- Scottish distribution and trends: The digital birds of Scotland (Forrester et al., 2012); and
- Scottish white-tailed eagle population and future range modelling (Sansom et al., 2016).
AD/FID Quantitative disturbance distances
- The start of this section states if the species was included in Ruddock and Whitfield, (2007).
- Disturbance distance AD and FID values (presented in metres) contained in the BDR database are presented; references are provided in the current report and in the BDR database.
- Depending on the information available in the reference, measures of AD/FID may be presented as a single value, mean AD/FID, median AD/FID and/or range (minimum/maximum) of AD/FID values. One or several of these measures for each source of disturbance in each reference represents one record.
- Some references contain multiple AD/FID values for different sources of disturbance.
MAD and/or Buffer zone Quantitative distances
- MADs and buffer zones (presented in metres) contained in the BDR database are presented; references are provided in the current report and in the BDR database.
Ecology and non-quantitative information on disturbance responses
- A brief account of the ecology of each species is provided.
- Non-quantitative information on disturbance response was used to assess sensitivity to disturbance when quantitative data were lacking or assessed as being of poor quality. References are provided in the text and at the end of the report.
Likely sensitivity to disturbance, quality of quantitative information and buffer zone suggestion
- A summary of the sensitivity to human disturbance, the quality of quantitative data and a suggested buffer zone to protect from human disturbance during the breeding and nonbreeding seasons is provided.
Knowledge gaps
- Reference to what data are unavailable for each species.
Results – Species accounts
A summary of each bird species considered in this report is presented in Table 1, information includes: likely sensitivity to disturbance, quality of the quantitative information held within the BDR database and suggested buffer zones for the breeding (BR) and nonbreeding (NBR) seasons.
Buffer zones indicate the potential range of distances to protect the majority of birds from human disturbance; for more precise disturbance distances on a focal species, each assessment should be carried out on a site-specific basis.
Individual species accounts, summarising the data held for each species in the BDR database, are presented in Tables 2 to 66.
|
Species |
Likely sensitivity to disturbance |
Quality of quantitative information (AD/FID) |
Buffer zone (m) suggestions during the breeding (BR) and nonbreeding (NBR) seasons |
|---|---|---|---|
|
Whooper swan, Cygnus cygnus |
Medium |
Medium agreement Limited evidence |
NBR = 200-600m |
|
White-fronted goose, Anser albifrons |
High |
Medium agreement Limited evidence |
NBR = 500-1000m |
|
Bean goose, Anser fabalis |
Medium |
* Medium agreement Limited evidence |
NBR = 200-600m |
|
Pink-footed goose, Anser brachyrhychus |
High |
Low agreement Limited evidence |
BR ≤1000m NBR = 500-1000m |
|
Greylag goose, Anser anser |
Medium |
Medium agreement Limited evidence |
BR and NBR = 200-600m |
|
Barnacle goose, Branta leucopsis |
Low/Medium |
Medium agreement Medium evidence |
BR and NBR = 50-200m |
|
Common shelduck, Tadorna tadorna |
High |
Medium agreement Medium evidence |
BR and NBR = 100-400m |
|
Mallard, Anas platyrhynchos |
Low/Medium |
High agreement High evidence |
BR = 50-100m NBR ≥ 100m |
|
Gadwall, Anas strepera |
Medium |
Medium agreement Limited evidence |
BR and NBR = 100-200m |
|
Pintail, Anas acuta |
Medium |
Low agreement Limited evidence |
BR and NBR = 100-200m |
|
Shoveler, Anas clypeata |
Medium |
Medium agreement Limited evidence |
BR and NBR = 100-200m |
|
Eurasian wigeon, Anas penelope |
High |
Low agreement Medium evidence |
BR = 100-200m NBR = 200-500m |
|
Greater scaup, Aythya marila |
High |
Medium agreement Limited evidence |
NBR = 150-450m |
|
Common eider, Somateria mollissima |
Medium/High |
Medium agreement Medium evidence |
BR = 100-200m NBR = 200-500m |
|
Common scoter, Melanitta nigra |
High |
Medium agreement Limited evidence |
BR = 300-500m
|
|
Common goldeneye, Bucephala clangula |
High |
Low agreement Medium evidence |
BR = 100-150m NBR = 150-800m |
|
Capercaillie, Tetrao urogallus |
Medium/High |
Medium agreement Medium evidence |
BR (nesting females) and NBR = 100m BR (lekking males) = 500-1000m NBR = 100m |
|
Black grouse, Tetrao tetrix |
Medium |
Medium agreement Medium evidence |
BR (nesting females) and NBR = 100-150m BR (lekking males) = 500-750m
NBR = 100m |
|
Red-throated diver, Gavia stellata |
High |
Medium agreement Medium evidence |
BR = 500-750m NBR = ≤1000m
|
|
Black-throated diver, Gavia arctica |
High |
Medium agreement Limited evidence |
BR = 500-750m NBR = ≤1000m
|
|
Great northern diver, Gavia immer |
Medium/High |
Medium agreement Medium evidence |
NBR = 100-350m
|
|
Slavonian grebe, Podiceps auritus |
Medium |
Low agreement Limited evidence |
BR and NBR = 150-350m |
|
White-tailed eagle, Haliaeetus albicilla |
High |
Low agreement Medium evidence |
BR = 500-1000m NBR = 250-500m
|
|
Osprey, Pandion haliaetus |
Medium/High |
Low agreement Medium evidence |
BR = 350-750m |
|
Golden eagle, Aquila chrysaetos |
High |
Low agreement Medium evidence |
BR = 750-1000m NBR = 250-500m
|
|
Red kite, Milvus milvus |
Medium |
Medium agreement Limited evidence |
BR and NBR = 150-300m
|
|
Marsh harrier, Circus aeruginosus |
Medium |
Low agreement Limited evidence |
BR and NBR = 300-500m
|
|
Hen harrier, Circus cyaneus |
Medium |
Medium agreement Limited evidence |
BR and NBR = 300-750m
|
|
Common buzzard, Buteo |
Low/Medium |
Medium agreement Medium evidence |
BR and NBR = 100-200m
|
|
Honey buzzard, Pernis apivorus |
Medium |
Medium agreement Limited evidence |
BR = 100-200m
|
|
Northern goshawk, Accipiter gentilis |
Medium |
Medium agreement Limited evidence |
BR = 300-500m
|
|
Kestrel, Falco tinnunculus |
Low/Medium |
Medium agreement Limited evidence |
BR = 100-200m NBR = ≤50m |
|
Eurasian hobby, Falco subbuteo |
Medium |
* Medium agreement Limited evidence |
BR = 200-450m
|
|
Peregrine falcon, Falco peregrinus |
Medium |
Medium agreement Limited evidence |
BR = 500-750m NBR = ≤200m
|
|
Merlin, Falco columbarius |
Medium |
Low agreement Limited evidence |
BR = 300-500m NBR = ≤200m
|
|
Eurasian oystercatcher, Haematopus ostralegus |
Medium |
Medium agreement Robust evidence |
BR = 50-100m NBR = 150-300m |
|
Ringed plover, Charadrius hiaticula |
High |
Medium agreement Medium evidence |
BR = 100-200m NBR = 100-300m
|
|
Grey plover, Pluvialis squatarola |
Medium |
Medium agreement Medium evidence |
NBR = 150-300m |
|
Golden plover, Pluvialis apricaria |
Medium |
Medium agreement Medium evidence |
BR and NBR = 200-500m |
|
Dunlin, Calidris alpina |
Medium |
Medium agreement Medium evidence |
BR = 100-200m NBR = 150-300m
|
|
Red knot, Calidris canutus |
Medium |
Medium agreement Medium evidence |
NBR = 100-300m |
|
Purple sandpiper, Calidris maritima |
Low/Medium |
No quantitative evidence |
BR and NBR <300m |
|
Wood sandpiper, Tringa glareola |
Medium |
High agreement Limited evidence |
BR = 150-300m |
|
Common redshank, Tringa totanus |
Medium |
Medium agreement Robust evidence |
BR = 100-200m NBR = 200-300m
|
|
Greenshank, Tringa nebularia |
Medium/High |
High agreement Robust evidence |
BR and NBR = 300-500m
|
|
Black-tailed godwit, Limosa limosa |
Medium |
Medium agreement Medium evidence |
BR and NBR = 100-200m |
|
Bar-tailed godwit, Limosa lapponica |
Medium |
Medium agreement Medium evidence |
NBR = 200-300m |
|
Eurasian curlew, Numenius arquata |
High |
Medium agreement Robust evidence |
BR = 200-300m NBR = 200-650m |
|
Whimbrel, Numenius phaeopus |
Medium |
Medium agreement Limited evidence |
BR and NBR = 100-300m |
|
Red-necked phalarope, Phalaropus lobatus |
Low |
No quantitative evidence |
BR <50m |
|
Little tern, Sternula albifrons |
Medium |
Medium agreement Limited evidence |
BR = 100-300m |
|
Sandwich tern, Thalasseus sandvicensis |
High |
No quantitative evidence |
BR ≥200m |
|
Common tern, Sterna hirundo |
Medium/High |
Medium agreement Medium evidence |
BR = 200-400m |
|
Arctic tern, Sterna paradisaea |
Medium |
Low agreement Limited evidence |
BR ≥200m |
|
Roseate tern, Sterna dougallii |
High |
Low agreement Limited evidence |
BR ≥200m |
|
Snowy owl, Bubo scandiacus |
Medium |
Low agreement Limited evidence |
NBR = 150-500m
|
|
Long-eared owl, Asio otus |
Medium |
Low agreement Limited evidence |
BR and NBR = 100-300m
|
|
Short-eared owl, Asio flammeus |
Medium/High |
Low agreement Limited evidence |
BR and NBR = 300-500m
|
|
Tawny owl, Strix aluco |
Low/Medium |
* Medium agreement Limited evidence |
BR = 50-200m NBR ≥50m
|
|
Barn owl, Tyto alba |
Low |
Medium agreement Limited evidence |
BR = 50-100m NBR ≥50m
|
|
Corncrake, Crex |
Medium |
Low agreement Limited evidence |
BR ≥100m |
|
European nightjar, Caprimulgus europaeus |
Medium/High |
Medium agreement Limited evidence |
BR = 150-500m |
|
Kingfisher, Alcedo atthis |
Low/Medium |
High agreement Limited evidence |
BR and NBR = 50-100m |
|
Crested tit, Lophophanes cristatus |
Low |
High agreement Limited evidence |
BR and NBR = 10-50m |
|
Crossbill species, Loxia spp |
Low |
Medium agreement Medium evidence |
BR and NBR = 50-200m |
* One or zero AD/FID record is available; degree of agreement is based on MAD records and/or non-quantitative information.
Species: Swans and geese
Whooper swan, Cygnus cygnus
Conservation Status
UK: Amber List, Schedule 1 European: Least Concern, Annex 1
UK status
Scarce Breeder, Winter Migrant
UK and Scottish population estimate
UK population = 28 breeding pairs, 19,500 individuals in winter (Woodward et al., 2020); Scottish population = 3-7 breeding pairs, 4,142 individuals in winter (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a strong increase in breeding birds (+488%) over 25 years.
Range increases of 35% and 16% of overwintering birds have been identified in Britain and Ireland respectively, consistent with an increase in the Icelandic breeding population (Balmer et al., 2013).
AD/FID Quantitative disturbance distances
Whooper swan was not included in Ruddock and Whitfield (2007).
Breeding season (Whooper swan):
Surveyor walking in a rural habitat in Denmark: FID = 155m (n = 1) (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID = 21.7m (n = 10) (Jiang and Møller, 2017).
Breeding season (Tundra swan, Cygnus columbianus, stand in species for Whooper swan):
Surveyor walking in Europe: FID = 78m (n = 1) (Jiang and Møller, 2017).
Nonbreeding season (Whooper swan):
Surveyor walking in Europe: FID = 155m (n = 1) (Møller, 2008a).
Nonbreeding season (Tundra swan, Cygnus columbianus, stand in species for Whooper swan):
Surveyor walking in Europe: FID = 200m (n = 1) (Møller, 2008a).
MAD and/or Buffer zone Quantitative distances
No MAD or buffer zones available for Whooper swan.
Ecology and non-quantitative information on disturbance responses
The Icelandic population of whooper swan overwinters exclusively in Britain and Ireland (Balmer et al., 2013). The highest densities are widespread in lowland areas of Scotland, northern and eastern England as well as Ireland; in Scotland and northern England the main notable absence is in highland areas (Balmer et al., 2013). Whooper swans overwinter in wetland areas including shallow, reed-fringed inland waterbodies in amongst grasslands and heaths or surrounded by forests or reedbeds, rivers, estuaries and shallow marine areas (Snow and Perrins, 1998). This species feeds almost entirely on aquatic vegetation in fresh and saline waters, but when this is not available, whooper swans will also forage in stubble fields and arable crops; increasingly, birds forage in flood lands and other wetlands in late winter and early spring (Snow and Perrins, 1998). Very few birds breed in the UK, some records stem from injured birds, although confirmed records in Shetland and the Outer Hebrides could reflect an expansion in breeding range (Balmer et al., 2013).
Whooper swans are known to be sensitive to human presence and “demands immunity from disturbance” (Snow and Perrins, 1998); several studies have shown that this species increases the time spent vigilant when disturbed (Rees et al., 2005; Black and Rees, 1984; Brazil, 1981). In China, several factors may have contributed to the decline in the number of whooper swans present during the breeding season; as well as factors to do with climate and habitat change, factors such as hunting, increased disturbance from tourists and an increase in human development projects (e.g. highways, mining, hydroelectric dam and oil field exploitation) have all contributed to the decline in the whooper swan population (Ma and Cai, 2002). In Scotland, the majority of deaths are from human-related causes, many due to collisions with overhead wires; this species is also susceptible to lead poisoning by ingesting spent gunshot (Forrester et al., 2012). Overwintering whooper swans in Scotland are known to adapt their activity patterns and foraging locations in response to disturbance, for example disturbance from farmers and dogs have led to abandonment of foraging areas and displacement between fields (Brazil, 1981).
However, whooper swans can habituate to some types of human activity, especially if the source of disturbance is predictable. In a study at Rongcheng Lake in China, an important wintering ground for migratory birds, Liu et al., (2018) found that overwintering whooper swans became less sensitive to human visitors feeding the birds as the daily disturbance frequency became higher or as the natural food supply depleted. In a similar study at the Black Cart floodplain in Scotland, Rees et al. (2005) found that the distance at which >5% of a flock of whooper swans became alert because of human activity decreased with the number of previous disturbance incidents in the day, indicating that the swans became less sensitive to disturbance events if daily disturbance frequency was high, although there was no evidence that habituation to disturbance persisted over long periods. Rees et al. (2005) also found that the time taken for the birds to resume undisturbed behaviour varied with the duration of the disturbance event, which in turn depended on the type of disturbance involved, with pedestrians alerting the birds for longer periods than vehicles and aircraft. Small numbers of whooper swans winter at Hogganfield Loch, Glasgow, where they join mute swans, ducks and geese that feed on bread and grain from the hand. Although whooper swans at this site are slightly less ‘tame’ than mute swans, they will come to within 1 m of people providing food (Bernie Zonfrillo, pers. comm.).
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Nonbreeding season buffer zone = 200-600m
Whooper swan is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for whooper swan, but the maximum FID value recorded for this species when approached by a pedestrian is 155m during both the breeding and nonbreeding seasons.
In the UK, whooper swan has the potential to be disturbed on roosting and foraging grounds during the nonbreeding season. Due to the scarcity of breeding whooper swans in the UK, this species is unlikely to be encountered on breeding grounds by humans. There are no published buffer zones for whooper swan, but from studies on geese, a minimum buffer zone of 200-600m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
Lack of studies measuring AD/FID for a range of sources of disturbance, and clear evidence that habituation can occur but apparently to very different extents at different sites.
White-fronted goose, Anser albifrons
Conservation Status
UK: Red List
European: Least Concern, Annex 1
UK status
Winter Migrant
UK and Scottish population estimate
UK white-fronted goose population = 0-1 breeding pairs, 14,000 individuals in winter (Woodward et al., 2020). Scottish population has declined since Forrester et al. (2012) estimated a wintering population of c.16,000 individuals.
UK long-term trend
The European subspecies (albifrons) breeding population has increased but distribution has shifted eastwards; winter population declines have been recorded at most sites in Britain although the range expanded by 36% between 1981/84 – 2007/11 (Balmer et al., 2013). The Greenland subspecies (flavirostris) continues to show a long-term decline in breeding numbers, winter numbers in Britain have declined since a peak in 1998/99 (Balmer et al., 2013; Forrester et al., 2012).
AD/FID Quantitative disturbance distances
Greenland white-fronted goose was not included in Ruddock and Whitfield (2007).
Nonbreeding season (Greenland white-fronted goose):
Hunting in Denmark: Min/Max FID = 200 to 500m (n = 400 to 600) (Fox and Madsen, 1997).
Nonbreeding season (tule greater white-fronted goose, Anser albifrons elgasi, stand in species for Greenland white-fronted goose):
Pedestrian (general) in the USA: Mean FID = 47m (n = 6); Min/Max FID = 25 to 100 (Ackerman et al., 2004).
MAD and/or Buffer zone Quantitative distances
No MAD or buffer zone available for white-fronted goose.
Ecology and non-quantitative information on disturbance responses
Two subspecies of arctic breeding white-fronted goose overwinter in the UK; the European (albifrons) subspecies which breeds in Russia winters mainly in southern England and the Greenland-breeding (flavirostris) subspecies winters mainly in Ireland and western and northern Scotland (Balmer et al., 2013; Wernham et al., 2002). In Britain, Islay and the Severn Estuary are two important overwintering sites (Balmer et al., 2013). In Scotland, numbers have declined in recent years due to chronic low productivity in the Greenland population; small foraging flocks on traditional peatland sites have been lost, coincident with a shift towards managed grasslands (Balmer et al., 2013). In the UK, white fronted geese forage in lowland areas including grasslands, arable fields and wetlands (Snow and Perrins, 1998)
This species is considered sensitive to human disturbance (Fox and Stroud, 2002; Forrester et al., 2012). Stroud et al. (2012) identified aircraft/helicopters, human disturbance of roost sites, and deliberate and accidental human disturbance from farmland feeding sites as likely to cause significant local, but not population-scale, impacts on Greenland white-fronted geese.
There is anecdotal evidence suggesting that this species avoids human activity more than other geese; for example, the flock that winters at southeast Loch Lomond is rarely seen from local roads because it tends to frequent fields that are not visible from roads (Fox et al., 2012). In contrast to that anecdotal observation, statistical analysis of detailed survey data on habitat use by Greenland white-fronted geese wintering in Islay found a tendency for goose numbers to be higher closer to roads (Griffin et al., 2020). However, that was thought likely to be due to counting bias (increased detection of goose flocks close to roads from vehicles used for these surveys). There was a very clear effect of shooting disturbance on the time-energy budgets of Greenland white-fronted geese on Islay (Griffin et al., 2020). Effects were proportional to the distance from the disturbance and became detectable where shooting occurred within ca. 800 m from Greenland white-fronted goose flocks. Greenland white-fronted goose flocks disturbed by shooting were prone to flushing, and when not flushed tended to reduce feeding time and increase vigilance for 3-5 minutes after the event (Griffin et al., 2020). The effect of shooting disturbance on Greenland white-fronted goose behaviour was much more acute than other causes of disturbance such as road or farm vehicles or birds of prey. Nevertheless, road vehicles were responsible for the largest numbers of flushes of Greenland white-fronted geese in Islay (Griffin et al., 2020). Marksmen vehicles caused particular disturbance, presumably because the geese learned to associate them with shooting (Griffin et al., 2020). Norriss and Wilson (1988) showed that disturbance has been an important factor affecting rates of population change in Ireland, with flocks with a restricted feeding range being more likely to suffer local population declines as a result of disturbance. Therefore, quantifying and reducing human disturbance of wintering Greenland white-fronted geese is recommended in the species action framework (Urquhart et al., 2015).
Likely sensitivity to disturbance = High
Quantitative information = Medium agreement & Limited evidence
Nonbreeding season buffer zone = 500-1000m
Greenland white-fronted goose is assessed to have a high sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for white-fronted goose, the maximum FID value recorded for this species when disturbed by hunting activities during the nonbreeding season is 500m.
In the UK, white-fronted goose has the potential to be disturbed on foraging and roosting grounds during the nonbreeding season. There are no published buffer zones for white-fronted goose, but from other studies on geese, a minimum buffer zone of 500-1000m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
There are very few published studies measuring AD/FID for white-fronted goose. Disturbance distance studies are required for a range of human activity for this species.
Bean goose, Anser fabalis
Conservation Status
UK: Amber List
European: Least Concern
UK status
Escaped Breeder, Winter Visitor
UK and Scottish population estimate
UK population = 230 (Taiga) individuals in winter (Woodward et al., 2020); Scottish population = c.250 individuals in winter, 10-100 during passage (Forrester et al., 2012).
UK long-term trend
Decreased considerably since early 20th century. Possibly increased slightly 1981-84 to 2007-11, but some local losses too (Balmer et al., 2013). Numbers in Scotland (mainly at Slamannan) increased between 1978 and 2004 (Forrester et al., 2012).
AD/FID Quantitative disturbance distances
Bean goose was not included in Ruddock and Whitfield (2007).
Nonbreeding season:
Hunting in Denmark: Min/Max FID = 200 to 500m (Fox and Madsen, 1997).
MAD and/or Buffer zone Quantitative distances
No MAD or buffer zone available for bean goose.
Ecology and non-quantitative disturbance responses
In Britain, bean geese (mainly the subspecies Taiga bean goose, Anser fabalis fabalis) overwinter in small numbers; the main concentrated wintering areas are on the Slamannan Plateau, Stirlingshire and in the Yare Valley, Norfork (Balmer et al., 2013) after migrating from breeding grounds across Western Siberia to Scandinavia (Wernham et al., 2002). Outside these main winter areas, the wintering range includes Orkney, Shetland, northeast Scotland, East Anglia, southeast and northwest England, although these areas may support few birds or birds for short periods only (Balmer et al., 2013). Bean geese forage on arable land, rough pasture and marshy areas (Snow and Perrins, 1998; Thom, 1986), mostly close to the coast, but also at some marshy inland sites (Balmer et al., 2013).
Bean geese were once a common winter visitor to Scotland, but numbers have fallen greatly since the early 20th century, in part due to changes in agriculture and climate changes (Thom, 1986), but increased human disturbance may play a role in the decline (BCM Environmental Services Limited, 2011).
Bean geese may be susceptible to hunting disturbance, although protected, in appearance they look similar to pink-footed geese (Thom, 1986). There are very few studies available investigating disturbance distances in this species, the upper disturbance for hunting activities has been reported to be 500m (Fox and Madsen 1997).
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Nonbreeding season buffer zone = 200-600m
Bean goose is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for bean goose, the maximum FID value recorded for this species when disturbed by hunting activities during the nonbreeding season is 500m.
In the UK, bean goose has the potential to be disturbed on foraging and roosting grounds during the nonbreeding season. A minimum buffer zone of 200-600m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
There are very few published studies measuring AD/FID for bean goose. Disturbance distance studies are required for a range of human activity for this species.
Pink-footed goose, Anser brachyrhychus
Conservation Status
UK: Amber List
European: Least Concern
UK status
Winter Migrant
UK and Scottish population estimate
UK population = 510,000 individuals in winter (Woodward et al., 2020);
Scottish population = 200,000 individuals in October, 100,000-150,000 individuals in winter/spring (Forrester et al., 2012).
UK long-term trend
There has been a strong increase in the winter population (Balmer et al., 2013). Population increased from 90,000 in 1981/84 to 360,000 in 2007/11 (Balmer et al., 2013) and this increased to 510,000 in 2015/16 (Woodward et al., 2020). The British range doubled in size between 1981/84 – 2007/11 (Balmer et al., 2013).
AD/FID Quantitative disturbance distances
Pink-footed goose was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: Mean FID = 61m (n = 4); Min/Max FID = 43 to 78m (Díaz et al., 2021).
Surveyor walking in tundra habitat in Svalbard: Range of mean FID = 41.7 to 175.0m (n = 24) (Madsen et al., 2009).
Migratory season:
Hunting in a farmland habitat in Denmark: Range of mean FID decreased from 500 to 350m following the closure of the hunting season (Madsen, 1985).
Nonbreeding season:
Hunting in a nearshore habitat in Denmark: Min/Max FID = 350 to 500m (n = 400 to 600) (Fox and Madsen, 1997).
MAD and/or Buffer zone Quantitative distances Breeding season:
Surveyor walking in tundra habitat in Svalbard: Buffer zone = 1000m (Madsen et al., 2009).
Ecology and non-quantitative disturbance responses
Pink-footed geese breeding in Iceland and eastern Greenland, migrate almost exclusively to Britain to overwinter (Balmer et al., 2013). Large concentrations of feeding and roosting flocks are recorded along the east coast and central-eastern lowlands of Scotland, Solway Firth as well as in a broad band across England from Lincolnshire to Norfolk with the highest densities close to the coast (Balmer et al., 2013). In the spring, this species migrates north back to breeding grounds, flocks stage in central and northern Scotland which accounts for large numbers of nonbreeding records recorded in April and early May (Balmer et al., 2013). Pink-footed geese generally avoid upland areas, this species favours foraging areas on flat intensively farmed lowland areas (e.g. improved or fertilised grasslands, stubble fields, pastures and newly sown cereal fields) but will also feed on extensive areas of saltmarsh in estuaries (Balmer et al., 2013; Snow and Perrins, 1998).
Pink-footed geese are sensitive to disturbance (JNCC, 2012) and there is potential for disturbance at roost sites in the winter which may shift locally in response to disturbance (Mitchell and Hearn, 2004). Overwintering roost sites in the UK include estuaries, large lakes and reservoirs, usually close to feeding grounds (Snow and Perrins, 1998). In Scotland, favoured winter daytime roosting sites include estuarine mudflats, lochs and reservoirs (Forrester et al., 2007). On foraging grounds on arable fields, pink-footed geese are highly responsive to disturbance from surrounding roads (Gill et al., 1996). A paper reviewed by Korschgen and Dahlgren, (1992) recorded that pink-footed geese were disturbed at a distance of 500m when more than 20 cars per day used a road during autumn; it was also noted that as few as 10 cars per day affected habitat use by geese and a buffer zone of 500m was suggested to render habitat acceptable to flocks of pink-footed geese.
Mitchell and Hearn (2004) have found that the main determinant of roost choice is lack of human disturbance, especially hunting disturbance; other factors such as exposure, shoreline vegetation, including trees, and availability of grazing appear to be unimportant. Hunting is known to alter the distribution of pink-footed geese; in the major staging areas in Denmark, disturbance from hunting can result in the emigration of almost the entire population to the Netherlands within one day (see Väänänen, 2001 for review).
Likely sensitivity to disturbance = High
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone ≤1000m
Nonbreeding season buffer zone = 500-1000m
Pink-footed goose is assessed to have a high sensitivity to human disturbance.
The maximum FID value recorded for pink-footed goose is 500m when disturbed by hunting activities during the nonbreeding season. The maximum FID value recorded during the breeding season is a mean of 175m when approached by a pedestrian. A buffer zone of 1000m has been reported to protect pink-footed geese from pedestrian disturbance.
In the UK, pink-footed goose has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. A buffer zone up to 1000m is suggested to protect nesting birds and a buffer zone of 500-1000m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
There are few published studies measuring AD/FID for pink-footed goose. Disturbance distance studies are required for a range of human activity for this species.
Greylag goose, Anser
Conservation Status
UK: Amber List, Schedule 1 – Part II
European: Least Concern
UK status
Introduced/Resident Breeder, Winter Migrant
UK and Scottish population estimate
UK population = 47,000 breeding pairs, 230,000 individuals in winter (Woodward et al., 2020); Scottish population = at least 25,000 native/naturalised birds present all year round, with a further 85,000+ arriving from Iceland to winter in Scotland in the early 2000s (Forrester et al., 2012), although that number of migrants has decreased in recent years.
UK long-term trend
Population has increased considerably between 1981/84 – 2007-11, much of the increase has been of the resident population (Balmer et al., 2013).
AD/FID Quantitative disturbance distances
Greylag goose was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: Mean FID = 180m (n = 4); Min/Max FID = 180 to 180m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Norway: Mean FID = 12.4 (n = 24); Min/Max FID = 6 to 20m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Poland: FID = 77 (n = 1) (Díaz et al., 2021).
Surveyor walking in an urban habitat in Poland: Mean FID = 50.8 (n = 2); Min/Max FID = 49 to 52.4m (Díaz et al., 2021).
Nonbreeding season:
Surveyor walking in a wetland habitat in Denmark: Range of mean FID = 171 to 230m (n = 7 to 24) (Bregnballe et al., 2009).
MAD and/or Buffer zone Quantitative distances
No MAD or buffer zone available for greylag goose.
Ecology and non-quantitative disturbance responses
Greylag geese are widespread in the UK both during the breeding and nonbreeding seasons; three populations occur in the UK (native Scottish, reintroduced and Icelandic populations) but ranges now overlap to such an extent that it is impossible to separate them (Balmer et al., 2013). The resident British/Irish greylag goose population is now widespread throughout England (except the southwest and in north and southwest Wales) and Scotland (except the uplands and northeast); resident birds are sedentary, breeding and nonbreeding distributions are similar (Balmer et al., 2013). Resident birds breed near wetlands and occasionally on ledges of steep rocky slopes and tall heather, especially in Scotland (Snow and Perrins, 1998).
The Icelandic greylag goose population breeds in Iceland and winters in Britain (with smaller numbers wintering in Ireland, Norway and the Faeroe Islands); the majority of Icelandic birds winter in Scotland particularly in Orkney, Caithness and in east-central Scotland, with smaller numbers in southern Scotland, England and Wales (Balmer et al., 2013; Wernham et al., 2002). All greylag geese prefer foraging areas on low-lying agricultural land (Balmer et al., 2013), but this species will also forage on grasslands as well as fresh or saline shallow water areas (Snow and Perrins, 1998). Greylag geese show a strong preference for large, open fields that offer a clear view of potential predators (Newton and Campbell, 1973) although smaller fields may be used during the winter (see Hearn and Mitchell, 2004 for review).
Greylag geese generally show more tolerance towards human disturbance compared with other geese species present in the UK; birds on breeding grounds, roosting sites and in foraging areas may tolerate some degree of disturbance (Díaz et al., 2021; Hearn and Mitchell, 2004). However, this species will move away from areas that have high levels of human activity such as roads and human habitation. Keller (1991), found that overwintering greylag geese were heavily impacted by roads; in northeast Scotland, birds were not found within 100m of the nearest road and the median distance was 400m. In the Netherlands, Feige et al. (2008) found that this species will not breed or forage within a minimum distance of 100m of human buildings.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 200-600m
Nonbreeding season buffer zone = 200-600m
Greylag goose is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for greylag goose when approached by a pedestrian is a mean of 180m during the breeding season and a mean of 230m during the nonbreeding season.
In the UK, greylag goose has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. There are no published buffer zones for greylag goose, but from other studies on geese, a minimum buffer zone of 200-600m is suggested to protect breeding and nonbreeding birds from pedestrian disturbance.
Knowledge gaps
There are few published studies measuring AD/FID for greylag goose. Disturbance distance studies are required for a range of human activity for this species.
Barnacle goose, Branta leucopsis
Conservation Status
UK: Amber List
European: Least Concern, Annex 1
UK status
Escaped Breeder, Winter Visitor
UK and Scottish population estimate
UK population = 1,550 breeding pairs, 105,000 individuals in winter (Woodward et al., 2020); Scottish population = 70,000 in winter (Forrester et al., 2012).
UK long-term trend
Prolonged increase in wintering numbers over recent decades (Balmer et al., 2013). The breeding range of the resident population has increased by 88% between 1988/91 – 2007/11; the growth of the Greenland population has also increased the number of overwintering birds (Balmer et al., 2013).
AD/FID Quantitative disturbance distances
Barnacle goose was not included in Ruddock and Whitfield (2007).
Breeding season (barnacle goose):
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 5 to 20.1m (n = 4) (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID 12.6m (n = 4) (Jiang and Møller, 2017).
Surveyor walking in tundra habitat in Svalbard: Range of Mean FID = 7.5 to 27.0m (n = 162) (Madsen et al., 2009).
Breeding season (brent goose, Branta bernicla, stand in species for barnacle goose):
Surveyor walking in a rural habitat in Denmark: FID = 20m (n = 1) (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID 23.5m (n = 6) (Jiang and Møller, 2017).
Nonbreeding season (brent goose):
Pedestrian (general) in a shoreline habitat in England: Min/Max AD = 23 to 150m (n = 45); Median FID = 51.5m; Min/Max FID = 5 to 178m (n = 89) (Liley et al., 2010).
MAD and/or Buffer zone Quantitative distances
No MAD or buffer zone available for barnacle goose.
Ecology and non-quantitative disturbance responses
Although small numbers of barnacle geese are resident in England and Wales, the majority of this species migrates from breeding grounds in Svalbard and Greenland to overwinter in the UK (Balmer et al., 2013; Wernham et al., 2002). The wintering populations of barnacle geese are widely distributed around the coasts, estuaries and wetland areas of the UK; birds recorded along the coast and islands of northwestern Scotland are largely from the Greenland-breeding population, whilst birds on the Solway Firth and on the east coast of Britain are largely from the Svalbard population (Balmer et al., 2013). Breeding and nonbreeding resident birds are more widely distributed and may also occupy inland areas, particularly in England (Balmer et al., 2013). This species feeds on grasslands grazed by farm animals or on autumn stubbles (Snow and Perrins, 1998), the overwintering migratory populations may feed in inland areas, but these are often within a few kilometres of their coastal wintering locations (Balmer et al., 2013).
Barnacle geese are regarded as vulnerable to human disturbance on breeding grounds (Madsen et al., 2009) as well as over hunting grounds during migration (Madsen and Fox, 1995). However, numbers of barnacle geese overwintering in the UK has increased rapidly over the last 40 years and this has resulted in conflict in agricultural areas (see Percival et al., 1997 for review). On Islay in Scotland, where approximately two-thirds of the East-Greenland breeding population overwinter, Percival et al. (1997) found that tactics to scare birds (e.g. people walking towards birds until they took flight, the use of gas guns and plastic tape) from an agricultural area, resulted in some birds moving towards undisturbed sites, but many individuals persisted in using the heavily disturbed sites, suggesting that some individuals and family groups have a high tolerance of disturbance on nonbreeding grounds.
Barnacle goose have become resident in parts of Sweden, including urban Stockholm. In this city barnacle geese live in public parks and feed on roadside verges and grass-covered roundabouts (Bob Furness pers. obs.). They show very little response to the presence of people, and have clearly habituated to this urban environment, illustrating the wide range of behavioural responses that are context-dependent.
Likely sensitivity to disturbance = Low/Medium
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 50-200m
Nonbreeding season buffer zone = 50-200m
Barnacle goose is assessed to have a low to medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are limited for barnacle goose. The maximum FID value recorded for barnacle goose when approached by a pedestrian is a mean of 27m during the breeding season; for brent goose, the maximum FID is 178m during the nonbreeding season.
In the UK, barnacle goose has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. There are no published buffer zones for barnacle goose, but from the range of published FID values, a buffer zone of 50-200m is suggested to protect breeding and nonbreeding birds from pedestrian disturbance.
Knowledge gaps
There are few published studies measuring AD/FID for barnacle goose specifically. Disturbance distance studies are required for a range of human activity for this species.
Species: Ducks
Common shelduck, Tadorna tadorna
Conservation Status
UK: Amber List
European: Least Concern
UK status
Migrant/Resident Breeder, Winter Visitor
UK and Scottish population estimate
UK population = at least 7,850 breeding pairs, 51,000 individuals in winter (Woodward et al., 2020); Scottish population = 1,750 breeding pairs, 7,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
The UK breeding range increased by 17% between 1981/84 – 2007/11, but the population increased only by 2% between 1995 – 2010; range increases are associated with the continued colonisation of inland breeding sites (Balmer et al., 2013). Increased winter ranges are consistent with breeding ranges, however, despite this, winter population trends in the UK and Ireland show shallow, steady declines since the mid-1990s (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Common shelduck was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 35 to 52m (n = 18), Min/Max FID = 18 to 70m (Díaz et al., 2021).
Nonbreeding season:
Surveyor walking over mudflats in Scotland: Mean FID = 178.4m (n = 22) (Dwyer, 2010).
Surveyor walking in Europe: Mean FID = 36.30m (n = 10) (Møller and Erritzøe, 2010).
Surveyor walking in Europe: Mean FID = 48.6m (n = 7) (Møller, 2008a).
Surveyor walking over mudflats in Denmark: Mean FID = 225m (n = 102), Min/Max FID = 55 to 700m (Laursen et al., 2005).
Pedestrian leisure (walking and watercraft) along the shoreline in England: Median AD = 50 (n = 3), Min/Max AD = 50 to 70m; Range of median FID = 40 to 62.5m (n = >6), Min/Max FID = 25 to 100m (Liley et al., 2011).
Pedestrian (general) along the shoreline in England: Median FID = 77.5m (n= 8), Min/Max FID = 50 to 140m (Liley et al., 2010).
Pedestrian walking/running on tidal flats in the Netherlands /Germany: Range of mean FID = 148 to 250m; Min/Max FID = 99 to 300m (Smit and Visser, 1993).
Non-motorised watercraft (kayak) in nearshore waters off Denmark: Mean FID = 220m (Laursen et al., 2017).
Non-motorised watercraft (wind surfer) in nearshore waters off Denmark: Mean FID = 400m (Laursen et al., 2017).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season:
Pedestrian walking/running along footpaths or the presence of railways close to intertidal areas in England: Buffer zone = 100m, although a buffer zone of 200m may be needed to protect a mix of intertidal species (Burton et al., 2002a)
Ecology and non-quantitative information on disturbance responses
In the UK, shelducks are found in most coastal regions where there is suitable lowland habitat (e.g. estuaries, muddy shores and coastal marshes) (Balmer et al., 2013); this species also increasingly breeds at inland sites (e.g. farmland, lakes, reservoirs and pig fields), particularly those in northern, central and southern England (Balmer et al., 2013). Shelducks feed mainly on salt-water molluscs when by the coast, but this species will also feed on aquatic invertebrates and plant material (Snow and Perrins, 1998). Breeding and nonbreeding distributions are similar; the highest concentrations of breeding shelduck are recorded along the East Anglian coastline, the Lancashire and Cumbrian marshes, the Uists and Orkney, as well as the area inland of the Wash extending into the Fens and Breckland (Balmer et al., 2013). Shelduck is generally a hole nesting species, nests are commonly located in tree hollows up to 8m above ground and mammal holes (e.g. rabbits) are also used; more rarely, this species may nest on the ground in the open or in dense vegetation up to 1km away from water (Snow and Perrins, 1998). Shelducks breeding in the UK do not migrate to an overwintering area, but the majority (≥90%) do have a well-defined moult migration to the Helgoland Bight of the Wadden Sea (Wernham et al., 2002). The moult migration starts as early as mid-June with birds gradually returning to the UK during mid-winter; a small number of birds remain in the UK to moult (Wernham et al., 2002).
Shelducks are potentially vulnerable to human disturbance, particularly during the moulting period when birds are completely flightless and are therefore more vulnerable to disturbance and predation (Salomonsen, 1968). Shelduck moulting areas are usually situated in places where there is relatively little disturbance, such as difficult to access mudflats (e.g. Meininger and Snoek, 1992; Bryant and Leng, 1975). Disturbance may also impact shelduck on their winter foraging grounds, Burton et al. (2002a) indicated that shelduck counts were significantly lower on English estuarine count sectors that were closer to footpaths, after curlew, shelduck was the second species most likely to take flight when disturbed by walkers. Burton et al. (2002a) also found that numbers of shelduck were reduced on count sections within 100m of railways, furthermore, Burton et al. (2002b) found that construction work around Cardiff Bay tended to reduce the densities of shelduck, although this tendency was not statistically significant in their study.
Although shelduck is not a quarry species, hunting is one of the principal causes of mortality in fledged shelducks in Scotland (Forrester et al., 2012). Forrester et al., 2012 identified a gap in current knowledge relating to human disturbance and shelduck and posed the question of whether the increase in breeding shelduck at inland sites is in response to human disturbance in coastal areas.
Likely sensitivity to disturbance = High
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 100-400m
Nonbreeding season buffer zone = 100-400m
Common shelduck is assessed to have a high sensitivity to human disturbance.
The maximum FID value recorded for common shelduck when approached by a pedestrian is 70m during the breeding season and 700m during the nonbreeding season, although generally FID values recorded during the nonbreeding season are less than 500m. For non-motorised watercraft, mean FID values up to 400m have been recorded during the nonbreeding season
In the UK, shelduck has the potential to be disturbed on breeding grounds as well as on moulting, foraging and roosting grounds during the nonbreeding season; as a hole nesting species shelduck may be less likely to be disturbed when on the nest. A buffer zone of 100-400m is suggested to protect both breeding and nonbreeding shelduck from pedestrian and boating disturbance, although a buffer zone at the lower end of this range may be sufficient to protect nesting birds during the breeding season.
Knowledge gaps
Further studies are required to record AD/FID during the breeding season. Limited information on buffer zones.
Mallard, Anas platyrhynchos
Conservation Status
UK: Amber List
European: Least Concern
UK status
Introduced/Resident Breeder, Winter Visitor
UK and Scottish population estimate
UK population = at least 61,000-145,000 breeding pairs, 675,000 individuals in winter (Woodward et al., 2020); Scottish population = 17,000-43,000 breeding pairs, 65,000-90,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
The UK breeding population increased by 20% between 1995-2010, range increased by 2% and 8% in Britain and Ireland respectively between 1988/91 - 2007/11 (Balmer et al., 2013). In contrast, although the range of wintering UK birds is similar to the breeding season, the wintering population has declined by 39% since around 1990 which is likely due to a reduction in overwintering European breeding migrants (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Mallard was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Scotland: Mean FID = 20m (n = 3), Min/Max FID = 4 to 28m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Spain: Range of mean FID = 10.8 to 20m (n = 19), Min/Max FID = 0.7 to 30.1m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Spain: Range of mean FID = 2.8 to 12m (n = 16), Min/Max FID = 1.4 to 12m (Díaz et al., 2021).
Surveyor walking in a rural habitat in France: Range of mean FID = 4.8 to 8m (n = 40), Min/Max FID = 3 to 15m (Díaz et al., 2021).
Surveyor walking in an urban habitat in France: Range of mean FID = 2 to 7.5m (n = 98), Min/Max FID = 0 to 13m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Hungary: Range of mean FID = 4.8 to 17.9m (n = 15), Min/Max FID = 2.4 to 28.6m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Hungary: Range of mean FID = 3.4 to 3.8m (n = 16), Min/Max FID = 0.6 to 8.3m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Czech Republic: Mean FID = 56.5m (n = 4), Min/Max FID = 38 to 68m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Czech Republic: Range of mean FID = 1 to 14m (n = 25), Min/Max FID = 0 to 15m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 12 to 57m (n = 70), Min/Max FID = 4 to 75m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Denmark: Range of mean FID = 5 to 11.1m (n = 29), Min/Max FID = 2 to 19m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Norway: Range of mean FID = 8.5 to 11.9m (n = 18), Min/Max FID = 4 to 18m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Norway: Range of mean FID = 4.5 to 6.1m (n = 38), Min/Max FID = 2 to 8m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Finland: Mean FID = 30m (n = 2) (Díaz et al., 2021).
Surveyor walking in an urban habitat in Finland: Range of mean FID = 6.5 to 7.9m (n = 9), Min/Max FID = 2 to 16m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Poland: Range of mean FID = 6 to 88m (n = 22), Min/Max FID = 0.7 to 98m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Poland: Range of mean FID = 3 to 73.9m (n = 30), Min/Max FID = 0.5 to 16.1m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Estonia: Range of mean FID = 19.1 to 38.3m (n = 4), Min/Max FID = 11.3 to 38.3m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Estonia: Range of mean FID = 4.1 to 6m (n = 10), Min/Max FID = 0.8 to 7.5m (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID = 9.9m (n = 339) (Jiang and Møller, 2017).
Nonbreeding season:
Surveyor walking over mudflats in Scotland: Mean FID = 162.52m (n = 7) (Dwyer, 2010).
Surveyor walking in Europe: Mean FID = 13.42m (n = 89) (Møller and Erritzøe, 2010).
Surveyor walking in Europe: Mean FID = 14.60m (n = 77) (Møller, 2008a).
Surveyor walking over mudflats in Denmark: Mean FID = 236m (n = 25), Min/Max FID = 60 to 400m (Laursen et al., 2005).
Surveyor walking in wetlands in Denmark: Range of mean FID = 108 to 195m (n = 5 to 188) (Bregnballe et al., 2009).
Surveyor walking in a range of habitats in Australia: Mean FID = 12.8m (n = 3) (Weston et al., 2012).
Pedestrian leisure (walking and watercraft) along the shoreline in England: Range of median FID = 30 to 40m (n = 3), Min/Max FID = 30 to 50m (Liley et al., 2011).
Pedestrian (general) along the shoreline in England: AD = 50 (n = 1); Median FID = 25m (n= 5), Min/Max FID = 10 to 50m (Liley et al., 2010).
Motorised watercraft (motorboat) in nearshore waters off Denmark: Mean FID = 110m (Laursen et al., 2017).
Motorised watercraft (motorboat) on a lake in Japan: Mean FID = 99.30m (n = 28) (Mori et al., 2001).
Non-motorised watercraft (inflatable boat) in nearshore waters off Denmark: Mean FID = 100m (Laursen et al., 2017).
Non-motorised watercraft (rowing boat) in nearshore waters off Denmark: Mean FID = 85m; Min/Max FID = 80 to 90m (Laursen et al., 2017).
Non-motorised watercraft (kayak) in nearshore waters off Denmark: Mean FID = 50m (Laursen et al., 2017).
Non-motorised watercraft (wind surfer) in nearshore waters off Denmark: Mean FID = 280m (Laursen et al., 2017).
Non-motorised watercraft (kite surfer) in nearshore waters off Denmark: Mean FID = 40m (Laursen et al., 2017).
Non-motorised watercraft (Sailing dinghy) on Brent Reservoir, England: Mean FID = 100m (Batten, 1977).
Non-motorised watercraft (pedestrian leisure) in a range of habitats and locations: Mean FID = 18m (Borgmann, 2012).
Drone (operated by a surveyor) in a zoo in France: Min/Max AD = 4 to 8m (n = 9); Min/Max FID = 4 to 8m (n = 4) (Vas et al., 2015).
Unknown season:
Surveyor walking around a lake in Pakistan: Mean FID = 27m (Mosvi et al., 2019).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season (Mallard):
Non-motorised watercraft (pedestrian leisure) in a range of habitats and locations: Buffer zone = 83m (Borgmann, 2012).
Nonbreeding season (Groups of dabbling ducks, Anas sp. including gadwall, mallard and pintail):
Pedestrian leisure boats in a range of habitats and locations: Buffer zone = 108m (Borgmann, 2012).
Ecology and non-quantitative information on disturbance responses
Mallard is a common, widespread and adaptable resident species in the UK; its absence is only notable in mountainous areas and non-aquatic habitats (Balmer et al., 2013). In the UK, this species is sedentary or dispersive over short distances, distribution is similar in both the breeding and nonbreeding seasons; the highest densities are found in lowland aquatic areas (Balmer et al. 2013; Wernham et al., 2002). Mallards inhabit a wide range of aquatic environments, large or small, including standing or flowing freshwater, ponds, canals, irrigation networks, sewage farms, brackish estuaries and shallow sheltered coastlines (Snow and Perrins, 1998). The breeding season can be greatly prolonged for this species, ground nests are usually concealed by vegetation, but birds will also nest under boulders, inside hollow trees and on man-made structures - nest boxes and baskets are readily used (Snow and Perrins, 1998).
In the winter, resident mallards are joined by European breeders which migrate south and west to overwinter in areas that include the UK (Wernham et al., 2002). Mallards are omnivorous and opportunistic with a wide diet consuming both plant and animal matter depending upon location and season; food can be obtained from water by pecking and sieving, dabbling and upending and also by grazing on land like geese or wigeon (Forrester et al., 2012; Snow and Perrins, 1998). This species will readily consume bread and other items offered by humans.
Mallards are known to be tolerant of humans and have adapted well to human environments; this species is a common occurrence on garden ponds, park lakes and sewage farms (see Woodward et al., 2015 for review). This species can habituate to human activity, especially if the source of disturbance is predictable, such as frequently used navigation routes used by boats or areas close to harbours (Platteeuw and Henkins, 1997). Mallards were considered to be one of the most tolerant species towards disturbance from water-based recreational activities on inland waterbodies in England and Wales (Tuite et al., 1984). Mallards have been noted to have shorter FIDs in response to an approaching human compared to other dabbling ducks, suggesting that they are more tolerant than the other members of the same family (Mori et al., 2001).
However, despite this species renowned tolerance of humans, habituation to human disturbance does vary between habitats; Díaz et al., 2021 showed that mallard FID values in urban habitats are generally lower than FID in rural habitats where human activity is likely to be much lower. During the breeding season, especially early on during incubation, mallards are known to be disturbed by humans. A literature review by Sinnott (2000) noted that in Montana, breeding mallards were more sensitive to disturbance from pedestrians and cyclists than from vehicles. In Iowa, disturbance from surveyors monitoring the use of artificial nests has been shown to cause a 10% nest abandonment rate (see Korschgen and Dahlgren, 1992 for review). A paper review by Korschgen and Dahlgren (1992) also noted that breeding mallards may be sensitive to disturbance from fishing activity; in Germany, the breeding stock of ducks (including mallard) at two small ponds declined by 85% due to disturbance from anglers and at the Seney National Wildlife Refuge in Michigan, mallards fail to nest in areas open to fishing.
The distribution of overwintering mallards in the UK is known to be strongly influenced by the presence of anglers (Cryer et al., 1987); as anglers and wintering ducks are attracted to the same limited areas, human presence can cause feeding or roosting birds to leave the area prematurely (Bell and Austin, 1985) which may have a detrimental effect on energy intake and expenditure (Knapton et al., 2000). Wildfowling disturbance on estuaries in the UK is also known to redistribute mallards (Madsen, 1994; Hirons and Thomas, 1993) and this species may congregate in refuge areas during the hunting season (see Sinnott, 2000 for review).
Likely sensitivity to disturbance = Low/Medium
Quantitative information = High agreement & High evidence
Breeding season buffer zone = 50-100m
Nonbreeding season buffer zone ≥ 100m
Mallard is assessed to have a low to medium sensitivity to human disturbance.
The maximum FID value recorded for mallard is 98m when approached by a pedestrian during the breeding season, although generally FID values recorded during the breeding season are less than 50m. The maximum FID value recorded during the nonbreeding season is 400m when approached by a pedestrian, although generally FID values are less than 200m; for motorised watercraft mean FID values of c.100m have been recorded and a range of mean FID values between 18-280m have been recorded for non-motorised watercraft.
In the UK, mallard has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. A buffer zone of 50-100m is suggested to protect nesting birds and a buffer zone ≥ 100m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian and boating disturbance.
Knowledge gaps
Mallard is relatively well studied, although the AD/FID values recorded during the breeding season is limited to one study.
Gadwall, Anas strepera
Conservation Status
UK: Amber List
European: Least Concern
UK status
Migrant/Resident Breeder, Winter Visitor
UK and Scottish population estimate
UK population = at least 1,250-3,200 breeding pairs, 31,000 individuals in winter (Woodward et al., 2020); Scottish population = 100-150 breeding pairs, fewer than 150 individuals in winter (Forrester et al., 2012).
UK long-term trend
The British breeding population increased by 83% between 1995 – 2010 corresponding with a large range expansion; in Ireland this is still a scarce breeding species (Balmer et al., 2013). UK wintering numbers also increased by 312% between 1983/84 – 2008/09 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Gadwall was not included in Ruddock and Whitfield (2007).
Breeding season (Gadwall):
Surveyor walking in Europe: FID = 55m (n = 1) (Jiang and Møller, 2017).
Nonbreeding season (Gadwall):
Pedestrian (general) along the shoreline in England: Min/Max FID = 50 to 60m (n= 2) (Liley et al., 2010).
Non-motorised watercraft (pedestrian leisure) in a range of habitats and locations: Mean FID = 65m (Borgmann, 2012).
Motorised watercraft (motorboat) on a lake in Japan: Mean FID = 64.5m (n = 19) (Mori et al., 2001).
Nonbreeding season (Groups of dabbling ducks, Anas sp. including gadwall, mallard and pintail):
Pedestrian leisure (general) in a range of habitats and locations: Mean FID = 100m (Borgmann, 2012).
Unknown season (Gadwall):
Surveyor walking around a lake in Pakistan: Mean FID = 20m (Mosvi et al., 2019).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season (Gadwall):
Commercial vehicle/machine (construction activity in England): Buffer zone = 200m (Wallis et al., 2019).
Nonbreeding season (Groups of dabbling ducks, Anas sp. including gadwall, mallard and pintail):
Pedestrian leisure boats in a range of habitats and locations: Buffer zone = 108m (Borgmann, 2012).
Ecology and non-quantitative information on disturbance responses
Gadwall is a resident species in the UK but is largely absent across much of Scotland, except in eastern Scotland, the Uists and Orkney (Balmer et al., 2013). Much of the current UK breeding population of gadwall is descended from an original breeding stock of wild caught birds at Dersingham Decoy, Norfolk around 1850, since this time the population has spread and now extends throughout much of the lowlands of central, eastern and northwest England (Balmer et al., 2013). The preferred habitat of gadwall is in lowland wetland areas that have fairly shallow, standing or slow-flowing open water with cover in the form of emergent vegetation, dry banks and islands; eggs are laid on the ground in a nest that is formed of a slight hollow lined with vegetation (Snow and Perrins, 1998). The increase in the number of reservoirs and particularly gravel pits has aided the spread of this species in Britain (Balmer et al., 2013; Briggs et al., 2012).
After the breeding season, resident gadwalls are joined by winter migrants from Iceland and the near Continent; the distribution of UK birds is slightly wider during the nonbreeding season compared to the breeding season due to dispersal from natal grounds, more inland sites are used by overwintering birds (Balmer et al., 2013) and some passage birds pass through the UK to overwinter in France, Spain and the Mediterranean (Wernham et al., 2002). Gadwall is a herbivorous species feeding on aquatic plants, but birds will also occasionally graze on land and eat cereal grains (Snow and Perrins, 1998).
Gadwalls are potentially sensitive to human disturbance, especially in areas where there are high levels of recreational disturbance. In the Netherlands, Platteeuw and Henkins (1997) report that overwintering gadwall and shovelers will often fly away from recreational disturbance (including water sports, anglers and swimmers) “at several hundreds of meters”. A study in a national park the south-eastern Virginia which has a high level of human recreational disturbance indicated that out of seven species of dabbling ducks, gadwall was one of the species most sensitive to disturbance (Pease et al., 2005). These sorts of disturbance events can impact activity budgets as gadwalls will spend more time displaying alert activity in areas of disturbance rather than feeding or resting (Paulus, 1984). A study by Briggs et al. (2012) found that gadwall can alter their habitat use in response to disturbance; birds have been shown to adjust their site preferences and patterns of site use in response to human disturbance in the southwest London area and consistently avoid areas where there is a high level of disturbance (e.g. water-skiing).
However, gadwall response to human disturbance varies. Mori et al. (2001) found that gadwall responded to pedestrian approach at relatively short distances in single-species flocks compared with some other wildfowl species. Conomy et al. (1998) found that gadwall were generally not disturbed by aircraft activity in North Carolina.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 100-200m
Gadwall is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for gadwall when approached by a pedestrian is a mean of 55m during the breeding season and 60m during the nonbreeding season; for motorised and non-motorised watercraft, mean FID values of c.65m have been recorded during the nonbreeding season.
In the UK, gadwall has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. A minimum buffer zone of 100-200m is suggested to protect both breeding and nonbreeding gadwall from pedestrian and boating disturbance.
Knowledge gaps
Further studies are required to record AD/FID during the breeding season. Limited information on buffer zones.
Pintail, Anas acuta
Conservation Status
UK: Amber List, Schedule 1
European: Vulnerable
UK status
Resident/Migrant Breeder, Winter Visitor
UK and Scottish population estimate
UK population = 27 breeding pairs, 20,000 individuals in winter (Woodward et al., 2020); Scottish population = 20-30 breeding pairs, fewer than 4,000-4,500 (occasionally up to 9,000) individuals in winter (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a weak decrease in breeding birds (-45%) over 25 years.
The small UK breeding population decreased in range by 32% between 1968/72 – 2007/11, the number of confirmed breeding records has also declined (Balmer et al., 2013). In contrast, the wintering ranged increased by 34% between 1981/84 – 2007/11, this corresponds with a long-term increase in numbers wintering in Britain since the early 1970s, although there has been a decline since the mid-2000s which may be due to a shift in the core wintering range (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Pintail was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in Europe: FID = 34.8m (n = 1) (Jiang and Møller, 2017).
Nonbreeding season:
Pedestrian (general) along the shoreline in England: FID = 100m (n = 1) (Liley et al., 2010).
Surveyor walking in a range of habitats Sir Lanka: Mean FID = 49.7 (n = 17); Min/Max FID = 20 to 82m (Gnanapragasam et al., 2021).
Nonbreeding season (Groups of dabbling ducks, Anas sp. including gadwall, mallard and pintail):
Pedestrian leisure (general) in a range of habitats and locations: Mean FID = 100m (Borgmann, 2012).
Unknown season:
Surveyor walking around a lake in Pakistan: Mean FID = 25m (Mosvi et al., 2019).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season (Groups of dabbling ducks, Anas sp. including gadwall, mallard and pintail):
Pedestrian leisure boats in a range of habitats and locations: Buffer zone = 108m (Borgmann, 2012).
Ecology and non-quantitative information on disturbance responses
Pintail is a rare and localised breeder in the UK, main breeding clusters are located in Orkney, North Uist, Tiree, East Anglian coast and the Ouse Washes with a few isolated records elsewhere (Balmer et al., 2013). This species breeds on lowland wetlands which may be on coastlines; the nest (a slight hollow lined with vegetation) is on the ground in short vegetational cover (Snow and Perrins, 1998).
Overwintering pintail in the UK, or those that pass through on migration, come from widely dispersed breeding grounds that include Iceland, Fennoscandia and the Baltic States (Wernham et al., 2002). In the UK, wintering pintails aggregate in large numbers at relatively few sites; the Burry Inlet, South Wales and the Welsh Dee Estuary are key sites (Balmer et al., 2013). Pintail is an omnivorous species feeding on a wide variety of plant and animal materials (Snow and Perrins, 1998) birds show a preference for feeding in estuaries as well as marshes, floodplains, sheltered coastlands and agricultural areas (Balmer et al., 2013). Unlike most ducks, pintail have more nocturnal habits and tend to forage in the evenings or at night and they spend much of the day resting or roosting.
Pintail is potentially sensitive to disturbance. Due to the aggregated distribution of this species, it is vulnerable to localised, stochastic events; recreation/tourism disturbance of staging and wintering pintail is considered of significance in several countries (European Commission, 2007a). Pintails are sensitive to hunting pressures. In Greece, hunting activity can cause mass displacement of ducks from the most important feeding areas; pintails and shovelers may completely stop feeding on shooting days (summarised in Madsen and Fox, 1995). Management of hunting disturbance can influence local distribution and abundance; in Denmark, the establishment of refuge areas where hunting is banned has increased pintail numbers. Maximum counts increased from less than 100 to over 4,000 pintail at a single site (Ulvshale Nyord) (Madsen 1998b).
However, pintails are known to tolerate some human presence. For example, at a study site in Iberia, this species feeds in rice paddies at night and commutes to an adjacent reservoir to roost during the day (Parejo et al., 2019). In comparison to other species of dabbling duck, pintail in some situations may have a higher tolerance of human disturbance; a study in a national park in south-eastern Virginia, which has a high level of human recreational disturbance, indicated that out of seven species of dabbling ducks (American black duck, gadwall, mallard, American wigeon, shoveler and green-winged teal), pintail was the least sensitive to disturbance (Pease et al., 2005). In another study at a national wildlife refuge in New Mexico, which has high levels of ecotourism, Taylor et al. (2019) found that behavioural response to human disturbance depended on the energy reserves of pintail; during a cold winter pintail did not show a significant energetic response to disturbance, therefore the authors suggested that under cold conditions, energy was conserved for short-term survival rather than used to respond to disturbance.
Likely sensitivity to disturbance = Medium
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 100-200m
Pintail is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for pintail when approached by a pedestrian is a mean of 35m during the breeding season and 100m during the nonbreeding season.
In the UK, pintail has potential to be disturbed on breeding grounds and foraging areas, although human disturbance is more likely on roosting grounds during the nonbreeding season. A minimum buffer zone of 100-200m is suggested to protect both breeding and nonbreeding pintail from pedestrian disturbance.
Knowledge gaps
Further studies are required to record AD/FID during the breeding season. Limited information on buffer zones.
Shoveler, Anas clypeata
Conservation Status
UK: Amber List
European: Least Concern
UK status
Migrant Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 1,100 breeding pairs, 19,500 individuals in winter (Woodward et al., 2020); Scottish population = 260-390 breeding pairs, 400-750 individuals in winter, 1,100-1,600 individuals during passage (Forrester et al., 2012).
UK long-term trend
The overall range size increased by 36% between 1981/84 – 2007/11, the majority of these gains have been in Britain, particularly in Orkney (Balmer et al., 2013). Breeding numbers remained relatively stable between 1968/72 – 2007/11, some fluctuation in distribution is associated with availability of suitable breeding wetlands. Wintering numbers increased by 70% between 1983/84 -2008/09 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Shoveler was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in Europe: FID = 28m (n = 1) (Jiang and Møller, 2017).
Nonbreeding season:
Pedestrian (general) along the shoreline in England: Min/Max AD = 30 to 150m (n= 2), Min/Max FID = 15 to 100m (n = 3) (Liley et al., 2010).
Motorised watercraft (motorboat) on a lake in Japan: Mean FID = 114.2m (n = 12) (Mori et al., 2001).
Nonbreeding season (Australasian shoveler, Anas rhynchotis, stand in species for Northern shoveler):
Surveyor walking in a range of habitats in Australia: FID = 19.2m (n = 1) (Weston et al., 2012).
Unknown season:
Surveyor walking around a lake in Pakistan: Mean FID = 22m (Mosvi et al., 2019).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season:
Commercial vehicle/machine (construction activity in England): Buffer zone = 200m (Wallis et al., 2019).
Ecology and non-quantitative information on disturbance responses
Shovelers are relatively scarce and local breeders in the UK. This species is largely absent across much of Scotland, except in the central lowlands and in the Uists and Orkney (Balmer et al., 2013). Shovelers have a dispersed distribution in southern and eastern England, their preferred habitat is in lowland areas including floodplains, reservoirs and gravel pits with associated wetland areas and some coastal estuaries (Balmer et al., 2013; Briggs et al., 2012); key breeding sites include the Lower Derwent, Yorkshire and Ouse and Nene Washes (Balmer et al., 2013). This species is a ground nesting bird, often on grass or rushes close to water (Snow and Perrins, 1998).
Wintering shoveler ranges are similar to their breeding areas (Balmer et al., 2013). Birds wintering in the UK are likely to be a mix of some resident birds and continental breeders, although some UK breeding birds will migrate to overwinter off northwestern Europe and North Africa (Wernham et al., 2002).
High overwintering concentrations are found along major waterways such as the Severn Trent, Thames and Great Ouse (Balmer et al., 2013). Shovelers are omnivorous and have a specialised bill for filtering water to feed on plankton, molluscs, insects and plant matter (Snow and Perrins, 1998).
Shovelers are potentially vulnerable to human disturbance in their wetland breeding and wintering areas; this species has been shown to alter its habitat use in response to disturbance (Briggs et al., 2012). In a study in the southwest London area, Briggs et al. (2012) found that wintering shovelers inhabiting inland waterbodies avoided disturbed areas (e.g. those used for recreational watersports) and used alternative sites in the event of isolated disturbance events; shovelers in this area showed a preference for reservoirs with other areas of water nearby which may serve act as alternative refuges in the event of disturbance. Tuite et al. (1984) listed wintering shoveler as one of the wildfowl species more susceptible to disturbance from water-based recreational activities on inland waterbodies in England and Wales; the greatest disturbance can be caused by power boating, with coarse fishing, sailing and rowing also important. In the Netherlands, Platteeuw and Henkins (1997) report that overwintering shovelers and gadwall will often fly away from a disturbance event “at several hundreds of meters”. However, other studies suggest that shovelers may be less sensitive to disturbance than other species of duck, especially gadwall, which share similar habitats. Pease et al. (2005) found that shovelers showed a strong flight response to human disturbance (e.g. people walking, biking and vehicles), although this was likely because shovelers were often closest to the source of disturbance compared with other species of dabbling duck. A paper review by Korschgen and Dahlgren (1992) noted that breeding shovelers may be sensitive to disturbance from fishing activity; in Germany, the breeding stock of ducks (including shovelers) at two small ponds declined by 85% due to disturbance from anglers.
Shovelers are sensitive to hunting pressures. In Greece, shovelers and pintails may completely stop feeding on shooting days (summarised in Madsen and Fox, 1995) and in Denmark, the establishment of refuge areas where hunting is banned has almost doubled the autumn and winter national totals of shoveler and wigeon (Madsen, 1998b). Shovelers in Denmark usually leave early before the hunting season starts, but the creation of refuges has encouraged some birds to stay in the country for longer (Väänänen, 2001).
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 100-200m
Shoveler is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for shoveler when approached by a pedestrian is a mean of 28m during the breeding season and 100m (AD = 150m) during the nonbreeding season. A mean FID value of 114m has been recorded for shoveler when approached by watercraft during the nonbreeding season.
In the UK, shoveler has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. A minimum buffer zone of 100-200m is suggested to protect both breeding and nonbreeding shoveler from pedestrian and boating disturbance.
Knowledge gaps
Further studies are required to record AD/FID during the breeding season. Limited information on buffer zones.
Eurasian wigeon, Anas penelope
Conservation Status
UK: Amber List
European: Least Concern
UK status
Resident Breeder, Winter Visitor
UK and Scottish population estimate
UK population = 200 breeding pairs, 450,000 individuals in winter (Woodward et al., 2020); Scottish winter population = 76,000-96,000 individuals (Forrester et al., 2012). Scottish breeding population may have declined since Forrester et al. (2012) estimated 240-400 breeding pairs.
UK long-term trend
Changes in breeding distribution suggest a decline in the Scottish uplands and gains in the islands, but there is some uncertainty over changes in breeding numbers (Balmer et al., 2013). Winter range expanded by 27% in Britain between 1981/84 – 2007/11, in Ireland there has been a 6% increase in range despite reported declines in numbers since the mid-1990s (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Wigeon was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in Europe: Mean FID 9.5m (n = 3) (Jiang and Møller, 2017).
Surveyor walking in an urban habitat in Finland: Range of mean FID = 4 to 4.4m (n = 18), Min/Max FID = 1 to 9m (Díaz et al., 2021).
Nonbreeding season:
Pedestrian leisure (walking and watercraft) along the shoreline in England: Median FID = 60m (n = 6), Min/Max FID = 50 to 100m (Liley et al., 2011).
Pedestrian (general) along the shoreline in England: Min/Max AD = 30 to 125m (n = 8); Median FID = 75.5m; Min/Max FID = 20 to 100m (n = 22) (Liley et al., 2010).
Surveyor walking in Denmark: Mean FID = 269m (n = 42), Min/Max FID = 150 to 1000m (Laursen et al., 2005).
Surveyor walking over mudflats in Scotland: Mean FID = 151m (n = 7) (Dwyer, 2010).
Surveyor walking in wetland habitat in Denmark: Range of mean FID = 117 to 205m (n = 5 to 26) (Bregnballe et al., 2009).
Surveyor walking in Sir Lanka: Mean FID = 41.5 (n = 2); Min/Max FID = 27 to 56m (Gnanapragasam et al., 2021).
Surveyor on motorboat on a lake in Japan: Mean FID = 67.7m (n = 38) (Mori et al., 2001).
Non-motorised watercraft (hunting punt) in Denmark: Mean FID = 100m
Non-motorised watercraft (fishing boat) in Denmark: Mean FID = 200m
Non-motorised watercraft (wind surfer) in Denmark: Mean FID = 700m
(Fox and Madsen, 1997).
Non-motorised watercraft (kayak) in nearshore waters off Denmark: Mean FID = 230m (Laursen et al., 2017).
Non-motorised watercraft (motorboat) in nearshore waters off Denmark: Mean FID = 250m (Laursen et al., 2017).
Non-motorised watercraft (wind surfer) in nearshore waters off Denmark: Mean FID = 500m (Laursen et al., 2017).
Unknown season:
Surveyor walking around a lake in Pakistan: Mean FID = 36m (Mosvi et al., 2019).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season:
Pedestrian walking/running around Strangford Lough in Ireland: Buffer zone = 250m (Mathers et al., 2000).
Commercial vehicle/machine (construction activity in England): Buffer zone = 200m (Wallis et al., 2019).
Ecology and non-quantitative disturbance responses
In the UK, Eurasian wigeon is an uncommon and localised breeder on lowland freshwater areas; the main breeding areas are in northern Scotland (Fife to the eastern Highlands north to Sutherland and Caithness, the Northern Isles and the Uists), as well as in the Pennines in England (Balmer et al., 2013). This species breeds under the cover of coniferous or deciduous wooded areas, close to or potentially fairly distant from water (Snow and Perrins, 1998).
During the nonbreeding season, wigeons are much more widespread around the UK; resident breeders are joined by overwintering birds from Iceland, Fennoscandia and Russia and have a preference for coastal areas (Balmer et al., 2013). The highest concentrations of wintering wigeon are recorded in the Northern Isles, inner Moray Firth, parts of central Scotland, large river valleys and estuaries in southern and eastern England as well as lakes in the west midlands of Ireland (Balmer et al., 2013). During the nonbreeding season, wigeons generally roost on the coast close to feeding grounds. Wigeon are vegetarian feeding on a diet of leaves, stems and roots (Snow and Perrins, 1998). This species can feed both during the day and night; where the feeding grounds are subject to daytime disturbance the birds may spend the day on the roost (Owen and Williams, 1976).
In a study at Strangford Loch, North Eastern Ireland, Mathers et al. (2000) record that overwintering wigeons are sensitive to human disturbance (particularly walking pedestrians) while foraging which is limited by tidal patterns; the study concluded that disturbance could have contributed to the decline of wigeon in Strangford Loch, although it is probably not the only factor involved. Wigeons are vulnerable to hunting disturbance, Madsen and Fox (1995) report that mobile shooting punts can cause greater disturbance than stationary ones; wigeons disturbed for a second time by a mobile punt took 168 minutes to resume feeding whereas fishing boats caused 20 minutes of disturbance. As wigeons can spend most of the daylight hours foraging during the autumn and winter, Madsen and Fox, (1995) note that birds can lose up 25% of foraging time on days with repeated disturbance. On the Exe Estuary, Fox et al. (1993) noted that just one disturbance incident at the wrong time can deter birds from feeding until the next tidal cycle.
Likely sensitivity to disturbance = High
Quantitative information = Low agreement & Medium evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 200-500m
Eurasian wigeon is assessed to have a high sensitivity to human disturbance.
The maximum FID value recorded for wigeon when approached by a pedestrian is a mean of 9.5m during the breeding season and a mean of 269m (max FID = 1000m) during the nonbreeding season, although generally, mean FID values recorded for pedestrian disturbance are less 200m. Mean FID values recorded for wigeon when approached by watercraft during the non-breeding season range from 100 to 700m.
In the UK, wigeon has the potential to be disturbed on breeding grounds, although human disturbance is more likely on roosting and foraging grounds at the coast during the nonbreeding season. A buffer zone of 100-200m is suggested to protect nesting wigeon and a buffer zone of 200-500m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian and boating disturbance.
Knowledge gaps
Few studies specify habituation to disturbance when recording AD/FID during the nonbreeding season.
Greater scaup, Aythya marila
Conservation Status
UK: Red List; Schedule 1
European: Least Concern
UK status
Scarce Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 0-1 breeding pairs, 6,400 individuals in winter (Woodward et al., 2020); Scottish population = 4,000-8,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
Scaup population has weakly declined since a massive decline in Scottish wintering population in 1970s (Balmer et al., 2013; Forrester et al., 2012). The winter range did expand by 57% between 1981/84 – 2007/11, but numbers in Britain have generally declined since 1970, although numbers in Northern Ireland have shown a large increase (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Greater scaup was not included in Ruddock and Whitfield (2007).
No AD/FID distance available for scaup
Breeding season (pochard, Aythya ferina, stand in species for scaup):
Surveyor walking in a rural habitat in Denmark: FID = 10m (n = 1) (Díaz et al., 2021).
Breeding season (tufted duck, Aythya fuligula, stand in species for scaup):
Surveyor walking in a rural habitat in Denmark: FID = 10.7m (n = 34), Min/Max FID = 8 to 14 (Díaz et al., 2021).
Surveyor walking in a rural habitat in Finland: FID = 28m (n = 2), Min/Max FID = 26 to 30 (Díaz et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season (Scaup):
Surveyor walking around inland waterbodies in the USA: Mean MAD = 146.4m (Trulio and White, 2017).
Watercraft (recreational boating) along the Mississippi river in the USA: Buffer zone = 450m (Havera et al., 1992).
Ecology and non-quantitative disturbance responses
In the UK, greater scaup is a very scarce breeder. This species mainly breeds on Arctic and sub-Arctic tundra; the breeding range in Europe stretches from western Siberia through European Russia to northern Fennoscandia and Iceland (Balmer et al., 2013; Wernham et al., 2002). In the past there have been several breeding records in Scotland particularly in base-rich or brackish waters in Orkney and the Outer Hebrides, but none since at least 1989 (Forrester et al., 2012; Snow and Perrins, 1998). The last confirmed breeding record was in Ireland (Co. Armagh) in 1999 (Balmer et al., 2013).
In the nonbreeding season, greater scaup winter on shallow coastal waters generally less than 10m deep (especially in the vicinity of sewage outlets), as well as sheltered bays, estuaries and brackish waters; it can also be found inland on large lakes and reservoirs (Snow and Perrins, 1998). The greatest numbers of wintering birds are found along the coast of northern and western Britain as well as northeastern and southwestern Ireland, wintering strongholds include the Dee, the Solway Firth, Loch Ryan, Ayrshire coast, Islay, the Firth of Forth and the Moray Firth and Lough Neagh (Balmer et al., 2013). Scaup are omnivorous feeding predominantly on molluscs (Snow and Perrins, 1998) mainly at night and they tend to flock together to roost on the sea during the day (Marchowski et al., 2015; Rare Breeding Birds Panel, 2020a).
The number of wintering scaup in the EU underwent a very large decline (> 50%) between 1990-2000, the reasons for this decline are largely unknown, but human disturbance is suspected to be important (European Commission, 2009). Increased disturbance from recreational activities from 1990 onwards may have reduced the amount of available wintering habitats, especially daytime roosts (European Commission, 2009). In the UK, human disturbance has been identified as one of the key threats to this species (Furness, 2016) and scaup at sea have been identified as having a high vulnerability to disturbance by boats (Furness et al., 2013). Mendel et al. (2008) has also identified scaup as highly sensitive to human disturbance and boat activity in coastal areas. During migration to and from breeding grounds, Knapton et al. (2000) found that mixed species flocks of diving ducks, including greater scaup, feeding on staging grounds at Lake Erie in North America, are frequently disturbed by human activity. Havera et al. (1992) suggest that during spring and autumn migration, minimum buffer zones of 450m should be used to protect rafting diving ducks from boating activity.
Likely sensitivity to disturbance = High
Quantitative information = Medium agreement & Limited evidence
Nonbreeding season buffer zone = 150-450m
Scaup is assessed to have a high sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for greater scaup. Studies measuring FID on other Aythya species (pochard and tufted duck) suggest that flushing distance is relatively low (<50m) during the breeding season and a buffer zone of 450m has been reported to protect migrating scaup from watercraft disturbance.
In the UK, scaup has the potential to be disturbed on roosting and foraging grounds at the coast during the nonbreeding season. Due to the scarcity of breeding scaup in the UK, this species is unlikely to be encountered on breeding grounds by humans. A buffer zone of 150-450m is suggested to protect roosting and foraging scaup during the nonbreeding season from pedestrian and boating disturbance.
Knowledge gaps
Lack of studies providing AD/FID for a range of disturbance types during the nonbreeding season.
Common eider, Somateria mollissima
Conservation Status
UK: Amber List
European: Endangered
UK status
Resident Breeder, Winter Visitor
UK and Scottish population estimate
UK population = 37,000 breeding pairs, 86,000 individuals in winter (Woodward et al., 2020); Scottish population = 20,000 nesting females, 64,500 individuals in winter (Forrester et al., 2012).
UK long-term trend
The distribution of breeding eiders has changed in the UK over the last 50 years. The breeding population increased in northwest Wales, Morecambe Bay and the Isle of Man between 1968/72 – 2007/11; in Northern Ireland, the population was ten times greater between 1977 – 2009 (Balmer et al., 2013). However, in western Scotland and Shetland, the population size and range has decreased (possibly as a result of predation, conflict with mussel farms and oil-pollution); declines in breeding numbers have also been noted elsewhere in Europe (Balmer et al., 2013). The overall winter range size has remained largely unchanged between 1981/84 – 2007/11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Common eider was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in Europe: Mean FID = 51.3m (n = 4) (Jiang and Møller, 2017).
Surveyor walking towards nest site in the Canadian Arctic: Mean FID = 16m (n = 69), Max FID = 70m (Mallory, 2016).
Nonbreeding season:
Motorised watercraft (high speed ferry service route) in the southern Kattegat Sea, Denmark: Min/Max FID = 0 to 1000m (n = 969) (Larsen and Laubek, 2005).
Motorised watercraft (large commercial fishing ship) in the German North Sea: Median FID = 208m (n = 154), Maximum FID = 3200m (Schwemmer et al., 2011).
Motorised watercraft (surveyor approaching moulting eiders in a motorboat) in nearshore waters around Norway: Mean AD = 330m (n = 48), Min/Max AD = 150 to 600; Mean FID = 177m (n = 48), Min/Max FID = 30 to 400m (Dehnhard et al., 2020).
Aircraft (helicopter) flying over males and nonbreeding females close to a gravel runway in the Canadian Arctic: Mead FID = 500m (Mallory, 2016).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Motorised watercraft (motorboat) around small offshore islands in Sweden: Buffer zone = 200m (Gotmark et al., 1989).
Ecology and non-quantitative information on disturbance responses
Eiders are seaducks associated with marine habitats during both the breeding and nonbreeding seasons; UK breeding birds are at the southernmost edge of the species’ Arctic range (Wernham et al., 2002). In the UK, breeding eiders are mainly recorded around the coast in northern areas including: most of Scotland, northern England, Isle of Man, North Wales and Northern Ireland (Balmer et al., 2013). This ground nesting species favours shoreline habitats and islands, but some birds are known to nest up to 3km inland (Snow and Perrins, 1998). The nest is composed of a slight hollow lined with available material, and large quantities of small feathers and down, and is often under the shelter of a rock or vegetation (Snow and Perrins, 1998).
Eiders in the UK are generally sedentary or disperse only short distances between breeding and nonbreeding grounds. During the nonbreeding season, birds located in eastern coastal areas may be joined by some overwintering continental eiders (Wernham et al., 2002). In the winter, eiders may be found around much of the coastline of Britain with the exception of the Solway Firth, Cardigan Bay and the Bristol Channel; the highest concentrations are to be found in northern areas (Balmer et al., 2013). All year round, eiders feed very close to the coast in water up to 3m deep, primarily on molluscs and crustaceans (Snow and Perrins, 1998), although this species roosts in open water away from feeding areas in shallow water (Merkel and Mosbech, 2008) where they are less likely to be disturbed.
Common eiders are able to habituate to some types of human activity (e.g. pedestrians and aircraft) and this species can tolerate relatively high levels of human disturbance. During the breeding season, incubating female eiders can sit tightly on the nest, for example, on Craigleith Island in Scotland, some females will allow pedestrian approach to within 1-2m before flushing, although other individuals will flush at a greater distance (Goodship 2021, pers. obs.). On the Mingan archipelago in Canada, Bolduc and Guillemette (2003) found that eider nesting success was not impacted by the frequency of human visitors, but the timing of visits was important to avoid exposing eggs to predators. In Norway, Stein and Ims (2016) have shown that the absence of eiders from nests due to human disturbance can increase egg predation risk by a factor of 6.42 for an increase of one additional daily disturbance. Bolduc and Guillemette (2003) suggested that researchers and wildlife managers should visit eider colonies as late as possible and avoid visiting colonies associated with high densities of eider egg predators. On Nasaruvaalik Island in the Canadian High Arctic, Mallory (2016) found that female eiders breeding next to a gravel runway allowed the wings of an aircraft to pass over them while still remaining on the nest. Dierschke et al. (2016) have found that the presence of offshore wind farms does not affect eider distribution.
However, boating activity, particularly boats that are moving quickly through eider foraging, roosting and moulting areas, have been shown to cause disturbance. In a study on wintering eider in southwest Greenland, Merkel et al. (2009) found that disturbance from boats could reduce foraging activity by up to 60% on a daily basis; eiders attempted to compensate for lost feeding opportunities by feeding more often, moving to sub-optimal foraging locations and switching to night-time feeding. Responses to boats may be especially strong in Greenland because this species is hunted from boats there. Jarrett et al., 2018 found that eider flight activity increases in the presence of marine activity including slow vessels/craft (including motorised and non-motorised boats for pleasure and commercial activities) and fast powerboats. The same authors found that eiders have a very low response rate within the 200-300m distance band from a passing ferry (eiders favour swim responses over flight or dive responses) and that the likelihood of eider flying away from passing ferries increased strongly in rougher sea states (Jarrett et al., 2018). In Norway, Dehnhard et al. (2020) found that boats disturbed moulting eiders resulting in displacement up to 771m; although most flocks returned to pre-disturbance behaviour within 10 mins after the disturbance event, the authors suggested that disturbance from boats increased locomotion costs, displacement from accessible foraging habitat and/or time lost for foraging or resting.
Likely sensitivity to disturbance = Medium/High
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 200-500m
Common eider is assessed to have a medium to high sensitivity to human disturbance.
FID values for eider are wide ranging. The maximum FID value recorded for eider is 70m when approached by a pedestrian during the breeding season and 3.2km when approached by a large commercial fishing boat during the nonbreeding season. For motorised watercraft in nearshore waters, a maximum FID of 400m has been recorded during the nonbreeding season. A buffer zone of 200m has been reported to protect breeding eider from watercraft disturbance.
In the UK, eider has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. A buffer zone of 100-200m is suggested to protect nesting eider and a buffer zone of 200-500m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian disturbance as well as disturbance from watercraft in nearshore waters.
Knowledge gaps
More studies required to record AD/FID during the breeding season and for pedestrian activity on the beach during the nonbreeding season.
Common scoter, Melanitta nigra
Conservation Status
UK; Red List; Schedule 1
European: Least Concern
UK status
Resident/Migrant Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 52 breeding pairs in Scotland, 135,000 individuals in winter (Woodward et al., 2020); Scottish winter population = 25,000-30,000 individuals (Forrester et al., 2012). Scottish breeding population has declined since Forrester et al. (2012) estimated 95 breeding pairs.
UK long-term trend
Eaton et al. (2021) state a stable number of breeding birds (-22%) over 25 years.
Breeding numbers have decreased in Scotland and Ireland since 1995/1999. The breeding population in Northern Ireland became extinct in 1993 (Balmer et al., 2013). The winter range expanded by 39% in Britain and Ireland between 1981/84 and 2007/11.
AD/FID
Quantitative disturbance distances
FID update (Schwemmer et al., 2011) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in Scotland: Min/Max FID (incubating female) =c.2 to 20m (Dr L. Griffin, pers. obs.).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 40 to 310m (n = 2); Min/Max AD (80% opinion range) = <10 to 500m; Min/Max AD (90% opinion range) = 300 to 500m.
Range of median FID = 5 to 125m (n = 3); Min/Max FID (80% opinion range) = <10 to 300m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season:
Motorised watercraft (large commercial ship) in the German North Sea: Median FID = 804m (n = 210), Maximum FID = 3200m (Schwemmer et al., 2011).
Motorised watercraft (high speed ferry service route) in the southern Kattegat Sea, Denmark: Min/Max FID = 0 to 1000m (Larsen and Laubek, 2005).
MAD and/or
Buffer zone
Quantitative distances
No buffer zone update published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Safe working distance = 300 to 800m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Ecology and non-quantitative disturbance responses
The majority of common scoters breed in tundra habitats near freshwater bodies (Snow and Perrins, 1998). In the UK, this species only breeds in Scotland, where it is restricted to the Flow Country of Caithness and Sutherland, larger lochs in Inverness-shire and Perthshire, and to a few scattered loughs in western Ireland (Balmer et al., 2013). Most breeding sites are in remote moorlands where birds nest on the ground in long heather at least 10m from the water’s edge, but at Loch Lomond and on Islay this species breeds on wooded islands (Snow and Perrins, 1998; Thom, 1986). The diet of common scoter is mainly molluscs which are obtained by diving, but in fresh water habitats this species will also feed on aquatic insects and fish eggs as well as occasionally small fish and seeds (Snow and Perrins, 1998).
Due to the low numbers of breeding common scoters in Scotland and the remote habitats in which they are found, the potential for disturbance from human recreational activities during the breeding season is limited, however, connectivity of breeding sites for human access (by tracks and roads) and forestry activity around breeding lochs will increase the potential disturbance risk for this species. Common scoters are known to be strongly site faithful and may continue to attempt breeding at historical sites despite an increased risk of human disturbance (Robson, 2017).
Common scoters are considered to be sensitive to human disturbance during the breeding season, but the level of sensitivity of individual birds likely depends on the stage of the breeding cycle as well as exposure to and ability to cope with human presence; birds nesting in more remote areas may be more sensitive to disturbance. In breeding lochs in Scotland (west Inverness-shire, Perthshire and Islay), it has been noted that incubating female common scoters will mostly sit tight when approached by a surveyor (moving slowly and quietly) to a distance of c.2-5m, although females incubating at nests on islands or mainland heaths are sometimes more "jumpy” and will leave the nest when approached to within c.10-20m (L. Griffin, pers. comm.). Some individuals appear to be highly tolerant of human disturbance; in Islay, a common scoter has been noted to remain at the nest within 20-40m of noisy fishing and pedestrian activity (e.g. talking loudly, getting in and out of boats and picnicking activity), the same bird even allowed a surveyor to fit a camera at the nest and instead of flushing, pecked the surveyor on the hand (L. Griffin, pers. comm.).
The distance at which female common scoter will return to a nest also varies between individual birds. Generally, females will not return until people are at least c.100-200m distant from a nest, but this distance is greater if the nest is in a remote location. In areas where birds may be habituated to people, female common scoter will return to nests at shorter distances; for example, on an island in Loch Garry that is near a regular fishing/camping location and a fish farm jetty, females have been noted to return to nests within 50-70m, although they often access the island on the side away from the sight of people (L. Griffin, pers. comm.). Human activity taking place between foraging areas and nest sites may prolong common scoter returning to their nests. At Loch Gorm on Islay, it has been noted that boats present on the loch or people fishing from the shore may delay foraging common scoter on the loch from returning to their nest on the heathland. Birds disturbed in this way have been observed to fly over their nests but not land, or they may carry on feeding for longer until the source of disturbance has gone. However, the severity of this kind of disturbance is difficult to judge, as common scoter may forage for between one and six hours, and birds may not resettle on their nests even when there is no apparent source of disturbance (L. Griffin, pers. comm.).
Foraging and resting common scoter present on freshwater lochs have been noted to be relatively tolerant of human presence and tend to flush only if a boat approaches rapidly and straight at the birds or makes a sudden appearance from behind an island etc. Common scoters have been observed to continue foraging within c.50-300m of boats and anglers on the bank, but this distance depends on how loud the agents of disturbance are and whether or not the disturbance is from one or multiple directions (L. Griffin, pers. comm.). Common scoter further away may be inquisitive and are known to approach slow moving boats, but if bird watchers with scopes for example approach to within <100m, common scoters tend to gently move a bit further away by "swim-feeding" (L. Griffin, pers. comm.).
Outside the breeding season, common scoter is rarely seen on land. Although this species may use freshwater lakes on migration, the majority of birds moult and overwinter at sea. They are present around much of the UK coastline, although patchily distributed in western Scotland and northwest Ireland (Balmer et al., 2013). The highest wintering concentrations are recorded in the Moray Firth, the coast from Angus south to County Durham, off Norfolk, Carmarthen Bay and the Irish Sea and off the South West coast of Ireland (Balmer et al., 2013). During the winter, common scoters roost communally at sea; they also periodically loaf on water during the day and, rarely, on islets or sandbanks (Cramp and Simmons, 1977).
Due to their distance from land during the nonbreeding season, the potential for human recreation disturbance is limited. However, common scoter is known to be particularly sensitive to human activities in marine areas including through the disturbance effects of ship and helicopter traffic (Garthe and Hüppop, 2004; Schwemmer et al., 2011; Furness et al., 2013; Furness and Wade, 2012; Bradbury et al., 2014; Kaiser et al., 2006). Common scoter may flush from boats that are over 3km away (Schwemmer et al., 2011) and this species is likely to be at risk of disturbance or displaced from habitats as a result of offshore wind turbines (Furness et al., 2013). Dierschke et al. (2016) reviewed all available evidence from operational offshore wind farms on the extent of displacement or attraction of seabirds in relation to these structures; a weak avoidance of offshore wind farms was noted for common scoter and velvet scoter (Melanitta fusca).
Likely sensitivity to disturbance = High
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 300-500m
Common scoter is assessed to have a high sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for common scoter, but the maximum FID value recorded for this species is 3.2km when approached by commercial shipping during the nonbreeding season. Although there are no official AD/FID values recorded for breeding common scoter, Dr Larry Griffin has personally noted that incubating female common scoter will flush from a nest when approached by a surveyor at a maximum approximate distance of 20m and that foraging birds on freshwater lochs will keep a maximum distance of 300m away from quiet boats and pedestrians. Ruddock and Whitfield (2007) recommended that a buffer zone of 300 to 500m would be required to prevent flushing from the nest during the breeding season.
Buffer zone to protect common scoter from forestry operations in the UK range from 300 to 800m during the breeding season.
In the UK, common scoter has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 300-500m is suggested to protect nesting common scoter during the breeding season from pedestrian and boating (on breeding lochs) disturbance. For activities with a high potential for visual and audial disturbance (e.g. forestry operations), a buffer zone ≤800m may be necessary. In marine areas during the nonbreeding season, a large buffer zone between 1 to 4km may be necessary to protect foraging and roosting birds from shipping disturbance.
Knowledge gaps
Lack of studies recording AD/FID during the breeding season.
Common goldeneye, Bucephala clangula
Conservation Status
UK: Red List; Schedule 1–Part II
European: Least Concern
UK status
Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 200 breeding pairs, 21,000 individuals in winter (Woodward et al., 2020); Scottish population = 150 breeding pairs, 10,000-12,000 in winter (Forrester et al., 2012).
UK long-term trend
UK breeding numbers increased from 13 to 38 between 1988/91 – 2007/11 and included colonisation of Perthshire and Aberdeenshire (Balmer et al., 2013). Wintering numbers have remained relatively stable between 1981/84–2007/11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
FID update (Díaz et al., 2021; Laursen et al., 2017; Borgmann, 2012; Liley et al., 2010) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Norway: FID = 18m (n = 1) (Díaz et al., 2021).
Surveyor walking in an urban habitat in Norway: Mean FID = 10.4m (n = 5); Min/Max FID = 6 to 22m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Finland: FID = 40m (n = 1) (Díaz et al., 2021).
Surveyor walking in an urban habitat in Finland: FID = 4m (n = 1) (Díaz et al., 2021).
Surveyor walking up to a nest box in Canada: Min/Max FID = 0.1 to <16m (Mallory et al., 1998).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 5 to 125m (n = 4 to 5); Min/Max AD (80% opinion range) = <10 to 300m; Min/Max AD (90% opinion range) = 150 to 300m.
Range of median FID = 5 to 75m (n = 5 to 8); Min/Max FID (80% opinion range) = <10 to 150m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season:
Pedestrian (general) along the shoreline in England: Min/Max AD = 75 to 100m (n = 3); Min/Max FID = 75 to 150m (n = 4) (Liley et al., 2010).
Pedestrian walking/running on Cannock Reservoir, England: Min/Max FID = 100 to 200m (Hume, 1976).
Non-motorised watercraft (sailing boat) on Cannock Reservoir, England: Min/Max FID = 350 to 400m (Hume, 1976).
Non-motorised watercraft (Sailing dinghy) on Brent Reservoir, England: Min/Max FID = 300 to 400m (Batten, 1977).
Non-motorised watercraft (pedestrian leisure) in a range of habitats and locations: Mean FID = 37m (Borgmann, 2012).
Non-motorised watercraft (sailing dinghy) in nearshore waters off Denmark: Min/Max FID = 300 to 400m
Non-motorised watercraft (rowing boat) in nearshore waters off Denmark: Mean FID = 360m
Non-motorised watercraft (sailing boat) in nearshore waters off Denmark: Mean FID = 360m
Non-motorised watercraft (kite surfer) in nearshore waters off Denmark: Mean FID = 740m
Motorised watercraft (motorboat) in nearshore waters off Denmark: Mean FID =640m
Motorised watercraft (jet-ski) in nearshore waters off Denmark: Mean FID = 765m, Min/Max FID = 700 to 830m
(Laursen et al., 2017).
Motorised watercraft (motorboat) on Cannock Reservoir, England: Min/Max FID = 550 to 700m (Hume, 1976).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (Borgmann, 2012) published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian (general): Buffer zone around active nests = 100-150m (Ruddock and Whitfield, 2007).
Forestry operations in the UK: Safe working distance = 150 to 300m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Nonbreeding:
Non-motorised watercraft (pedestrian leisure) in a range of habitats and locations: Buffer zone = 163m (Borgmann, 2012).
Ecology and non-quantitative disturbance responses
In Scotland, confirmed goldeneye breeding records are concentrated in Strathspey, Great Glen, River Dee and around Loch Tay; in England, confirmed breeding has been recorded in Northumberland and Avon (Balmer et al., 2013).
Goldeneye is a cavity nesting species with a preference for habitats around freshwater lakes, pools, rivers and deep marshes; this species will readily breed in nest boxes (Snow and Perrins, 1998; Dennis and Dow, 1984; Mallory and Weatherhead, 1993; Mallory et al., 1998). This species feeds during the daytime primarily on molluscs, crustaceans and insect larvae depending upon locality and season (Snow and Perrins, 1998). During the breeding season goldeneyes exhibit relatively low to moderate flushing distances in response to human disturbance, likely in part due to the lack of visual stimuli inside cavities (Ruddock and Whitfield, 2007; Mallory and Weatherhead, 1993; Mallory et al., 1998). In a study in Canada investigating female goldeneye nest defence, Mallory et al. (1998) found that 43% of female goldeneyes waited until the observer was on the tree before flushing and that this species flushed at closer distances as incubation proceeded. In Europe, Díaz et al. (2021) recorded low flushing distances (4 to 40m) in response to disturbance from a surveyor walking in the breeding season.
In the nonbreeding season, resident breeding goldeneye are joined by overwintering birds from Fennoscandia and Russian breeding grounds; they have a preference for coastal areas and a wide variety of freshwater habitats (Balmer et al., 2013). This species is widely distributed throughout Scotland and northern England with the exception of some upland areas; further south, winter distribution is patchy and focussed on suitable coastal areas, river valleys and wetland habitats (Balmer et al., 2013), they may also be found in the vicinity of sewage outfalls (Campbell and Milne, 1977). Goldeneye can be a gregarious flocking species, congregating at communal roost sites overnight (Snow and Perrins, 1998). Separate to their feeding grounds, goldeneyes roost on open water at the coast, on standing water or on rivers (Duncan and Marquiss, 1993). In some foraging and roosting areas goldeneye may be susceptible to human disturbance, especially from water-based leisure activities such as fishing and boating (e.g. Laursen et al., 2017; Tuite et al., 1984; Holloway, 1997; Hume, 1976; Campbell and Milne, 1977); disturbance from motorised watercraft can cause goldeneyes to flush over 800m away (Laursen et al., 2017). Goldeneye can also be sensitive to hunting pressures particularly during the winter when food may be scarce; in Ireland Evans and Day (2002) recorded that goldeneye moved away from the disturbed shorelines of Lough Neagh where hunting took place to central, relatively less disturbed areas of the Lough. In the Netherlands, Platteeuw and Henkins, 1997 considered goldeneye to be a particularly shy species, although goldeneye are generally not found in areas with high densities of recreation. However, not all wintering grounds are disturbed by human activity; in Orkney, goldeneye is largely present in very sheltered areas and inland lochs where marine activity is unlikely and therefore this species rarely comes into contact with marine activity in Orkney (Jarrett et al., 2018).
Likely sensitivity to disturbance = High
Quantitative information = Low agreement & Medium evidence
Breeding season buffer zone = 100-150m
Nonbreeding season buffer zone = 150-800m
Common goldeneye is assessed to have a high sensitivity to human disturbance.
The maximum FID value recorded for goldeneye when approached by a pedestrian is 40m during the breeding season and 200m during the nonbreeding season. For non-motorised watercraft mean FID values ranging between 37 to 740m have been recorded and mean FID values between 640 to 765m (max FID = 830m) have been recorded for motorised watercraft.
There are few suggested buffer zones for goldeneye. Ruddock and Whitfield (2007) suggested that a buffer zone of 100 to 150m would be required to prevent flushing from the nest during the breeding season. In the nonbreeding season, Borgmann, (2012) suggested a buffer zone of 163m to protect birds from non-motorised watercraft disturbance, but a larger buffer zone may be required for noisy activities in heavily disturbed areas.
In the UK, goldeneye has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season; as a hole nesting species, goldeneye may be less likely to be disturbed when on the nest. A buffer zone of 100-150m is suggested to protect nesting goldeneye and a buffer zone of 150-800m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian and boating disturbance.
Knowledge gaps
More studies required to record AD/FID during the breeding season and for pedestrian activity on the beach during the nonbreeding season.
Species: Grouse
Capercaillie, Tetrao urogallus
Conservation Status
UK: Red List, Schedule 1
European: Least Concern, Annex 1
UK status
Re-introduced Breeder
UK and Scottish population estimate
Scottish population only = 1,100 individuals in winter (Woodward et al., 2020); Forrester et al. (2012) suggest 300 lekking males in early 2000s, and a winter population of 1,300 to 2,800 individuals.
UK long-term trend
Eaton et al. (2021) state a strong decrease in breeding birds (-49%) over 22 years.
There was a 55% decrease in the number of occupied 10 km squares between 1981-84 and 2008-11 (Balmer et al., 2013). The population declined from about 20,000 birds in the 1970s, but declines have been partially mitigated in some areas by predator control and removal of fences on which collisions were occurring (Forrester et al., 2012).
AD/FID
Quantitative disturbance distances
FID update (Jiang and Møller, 2017; Thiel et al., 2007; Catt et al., 1998) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in Europe: FID 77.5m (n = 1) (Jiang and Møller, 2017).
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD for nesting females = 75m (n = 15); Min/Max AD (80% opinion range) = <10 to 150m; Min/Max AD (90% opinion range) = 100 to 150m.
Range of median FID for nesting females = 5 to 30m (n = 16); Min/Max FID (80% opinion range) = <10 to 100m.
Median AD for lekking males = 125m (n = 9); Min/Max AD (80% opinion range) = 100 to 750m; Min/Max AD (90% opinion range) = 500 to 750m.
Median FID for lekking males = 75m (n = 7); Min/Max FID (80% opinion range) = 50 to 500m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season:
Pedestrian (general) in a forest habitat in Europe: Mean FID = 27m (n = 752); Min/Max FID = 1 to 104m (Thiel et al., 2007).
Surveyor walking in a forest habitat in Scotland: Mean FID for males = 46m (n = 39)
Surveyor walking in a forest habitat in Scotland: Mean FID for females = 30m (n = 35)
(Catt et al., 1998).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (Coppes et al., 2017; Thiel et al., 2007) published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian leisure activity in forest land in Germany: Buffer zone = 800m (Coppes et al., 2017).
Forestry operations and recreational activities in Scotland:
Buffer zone for nests and broods = 100m
Forestry operations and recreational activities in Scotland:
Buffer zone for leks = 1000m
Buffer zone around leks for stalkers = 500 to 1000m (Kortland, 2006).
Forestry operations in the UK: Safe working distance = 200 to 800m (Currie and Elliot, 1997).
Forestry operations in Scotland: Safe working distance = 200 to 1000m (Forestry Commission Scotland, 2006).
Nonbreeding season:
Pedestrian (general) in a forest habitat in Europe: Buffer zone = 100m (Thiel et al., 2007).
Pedestrian leisure activity in forest land in Germany: Buffer zone = 800m (Coppes et al., 2017).
Ecology and non-quantitative disturbance responses
Capercaillie is a resident upland woodland species confined to pine forests in the north of Scotland (Balmer et al., 2013; Forrester et al., 2012). The main areas for this species include Easter Ross, Strathspey and Aberdeenshire, with only a few occupied sites outside of these areas; birds are largely sedentary, breeding and nonbreeding distribution ranges are similar (Balmer et al., 2013). Individual capercaillie normally use the same areas of summer and winter habitat in the same forest each year (Kortland, 2006). Mature conifer forests are typically used, especially Scots pine, open enough to support ground vegetation rich in dwarf shrubs (Forrester et al., 2012). Capercaillie is generally a ground nesting species, feeding on the ground in summer and mainly in the crowns of trees during winter (Snow and Perrins, 1998). Adults feed on plants including leaves, needles, stems, berries, mosses and rushes depending on the season; young chicks feed mostly on insects and spiders (Snow and Perrins, 1998). In winter, capercaillie live mostly in trees and eat conifer needles (Kortland, 2006).
Capercaillie populations in Scotland have declined significantly in the last 40 years. Reasons for the decline include loss of suitable habitat, unfavourable woodland management, climate change, predation, collisions with deer fences as well as disturbance (Kortland, 2006).
There is an increasing body of research that indicates that capercaillie stay away from areas where there is human activity. For example, in a study in the Spey valley in Scotland, Moss et al. (2014) investigated the impacts of human disturbance on capercaillie through the distribution of their droppings in relation to woodland tracks and entrances; droppings were found to be sparser within 300 to 800m of entrances and 70 to 235m of tracks, depending on track use and habitat. Moss et al. (2014) estimated that disturbance along the tracks deterred capercaillie from a belt of ground at least 140m wide and up to 470m long where people and dogs strayed off tracks. In another study by Summers et al. (2007) in the Cairngorms National Park, capercaillie avoided areas within 61 to 108m of public access tracks, the range being dependent on the level of pedestrian activity along the track. Capercaillie consistently disturbed away from foraging grounds may have fat reserves to survive only nine days (Hissa et al. (2003)). Kortland (2006) states that capercaillie can become habituated to predictable disturbance and will use habitat within 100m of tracks provided there is abundant screening and if walkers remain on the tracks; Kortland (2006) also states that if people or their dogs wander off tracks, capercaillie will stop using the areas where this happens.
The Capercaillie Biodiversity Action Plan Group (CBAPG) is responsible for implementing the Species Action Plan for Capercaillie on behalf of the UK Biodiversity Partnership. The current forest management for capercaillie builds on the Capercaillie Life project, which ran from 2002-2007 (Kortland, 2006). As recommended in Ruddock and Whitfield (2007), the guidance and management plans provided by the CBAPG should be followed in the UK. For survey work, NatureScot’s guidance on capercaillie survey methods should be followed (NatureScot, 2013).
Likely sensitivity to disturbance = Medium/High
Quantitative information = Medium agreement & Medium evidence
Breeding season (Nesting females) buffer zone = 100m
Breeding season (Lekking males) buffer zone = 500-1000m
Nonbreeding season buffer zone = 100m
Capercaillie is assessed to have a medium to high sensitivity to human disturbance.
The maximum FID value recorded for capercaillie when approached by a pedestrian is a mean of 77.5m during the breeding season and a mean of 46m (max FID = 104m) during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for capercaillie during the breeding season is 100-150m for nesting females and 500-750m for lekking males. Buffer zones to protect capercaillie during the breeding season from pedestrian activity and forestry operations range from 800 to 1000m; during the nonbreeding season, buffer zones range from 100 to 800m.
The data presented in this report are broadly consistent with the buffer recommendations detailed in the forest management guide for capercaillie issued by the CBAPG. The CBAPG recommends that forestry operations and known recreational activities etc should be avoided within 1km of lek sites between 1 March and 15 May. Deer control work is acceptable within 1km of leks between 1 March and 15 May, however, stalkers must stay at least 500m from lek sites between 4am and 9am. An exclusion zone of 100m must be used to prevent disturbance to nests and broods. Pedestrian disturbance must be avoided within 100m from tracks when passing though capercaillie habitat.
In the UK, capercaillie has the potential to be disturbed on breeding grounds as well as at roosting areas and foraging grounds during the nonbreeding season. The CBAPG recommends that a buffer zone of 500-1000m is used to protect leks and a buffer zone of 100m is used to protect nesting females to avoid pedestrian disturbance during the breeding season. Pedestrians should stick to paths when walking though capercaillie habitat at all times of the year and it is suggested that capercaillie habitat should not be disturbed within 100m.
Knowledge gaps
Lack of studies measuring AD/FID for pedestrian activity during the nonbreeding season.
Black grouse, Tetrao tetrix
Conservation Status
UK: Red List
European: Least Concern
UK status
Resident Breeder
UK and Scottish population estimate
UK population = 4,850 lekking males (Woodward et al., 2020); Scottish winter population = 7,500-19,000 individuals (Forrester et al., 2012). Forrester et al. (2012) estimated the Scottish population to be between 3,550-5,750 lekking males in the early 2000s, but population may have declined since that publication.
UK long-term trend
Declining in recent decades, especially latter part of 20th century, and range contracting; a 29% contraction in breeding range occurred between 1968/72 – 2007/11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
FID update (Díaz et al., 2021; Jiang and Møller, 2017; Schranz, 2009) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in the Ukraine: Mean FID = 24.3m (n = 6);
Min/Max FID = 20 to 28m (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID 24.3m (n = 6) (Jiang and Møller, 2017).
Surveyor walking over moorland in England: Range of mean FID = 74 to 86m (n = 44); Min/Max FID = 62 to 101m (Baines and Richardson, 2007).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD for nesting females = 5 to 75m (n = 8 to 11); Min/Max AD (80% opinion range) = <10 to 150m; Min/Max AD (90% opinion range) = 100 to 150m.
Range of median FID for nesting females = 5 to 30m (n = 8 to 11); Min/Max FID (80% opinion range) = <10 to 100m.
Median AD for lekking males = 225m (n = 17); Min/Max AD (80% opinion range) = 100 to 750m; Min/Max AD (90% opinion range) = 500 to 750m.
Median FID for lekking males = 225m (n = 17); Min/Max FID (80% opinion range) = 50 to 500m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season:
Surveyor walking over moorland in England: Range of mean FID = 17 to 88m (n = 107); Min/Max FID = 7 to 106m (Baines and Richardson, 2007).
Surveyor skiing in an alpine habitat in Switzerland:
Range of mean FID for males= 11.5 to 12m (n = 171); Min/Max FID = 1 to 80m
Range of mean FID for females= 8.1 to 11.3m (n = 77); Min/Max FID = 1 to 60m
(Schranz, 2009).
Pedestrian leisure activity (skiing and snow ploughs) in an alpine habitat in Bavaria:
Range of FID for black grouse under cover = <10 to 30m.
Range of FID for black grouse in the open = >30 to 100m.
(Zeitler, 2000)
MAD and/or
Buffer zone
Quantitative distances
Buffer zone updates (Arlettaz et al., 2013; Schranz, 2009) published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Safe working distance = 300 to 1000m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Nonbreeding season:
Pedestrian leisure (winter sports) in alpine habitats in Switzerland: Buffer zone = 120m (Arlettaz et al., 2013; Schranz, 2009).
Ecology and non-quantitative disturbance responses
Black grouse is a resident species in upland areas of Britain where it shows a preference for young plantations on moorlands, marginal farmland and woodland edges; as plantations mature, this habitat becomes less suitable and this may result in losses (Balmer et al., 2013). The highest abundance of this species has been recorded in upland areas of northern and central Scotland, the Southern Uplands, the Pennines and North Wales; birds are largely sedentary, and breeding and nonbreeding distribution ranges are similar (Balmer et al., 2013). Black grouse is generally a ground nesting species which feeds predominantly on plants; the main foods include buds, needles, pinecones, dwarf shrubs, grasses and berries, depending upon location and season (Snow and Perrins, 1998).
Disturbance caused by human recreational activities are considered to be a serious threat to grouse in central Europe (Storch, 2000). Disturbance in black grouse habitats can cause behavioural changes in the short-term and longer-term changes, in habitat use, spatial distribution and extinction of local populations (Storch, 2000; Zeitler 2000).
There is a growing body of evidence to show that recreational winter sports in the Alps causes disturbance to black grouse (Arlettaz et al., 2013; Schranz, 2009; Zeitler, 2000; Laiolo and Rolando, 2005; Baltic, 2005, Baltic et al., 2005). Zeitler found that black grouse kept distances of at least 150m away from new sources of disturbance such as newly operating snow generators and ski runs active outside the normal operational period. Under the cover of spruce or dwarf pines, Zeilter (2000) also found that this species can tolerate disturbances that occur within normal spatial and temporal patterns, but outside in the open, birds are more easily disturbed. Arlettaz et al. (2013) found that even moderate levels of disturbance, such as that caused by off-piste skiing activity, are enough to elicit a chronic stress response in black grouse. Compared with capercaillie, black grouse is a smaller species and may be more vulnerable to the risk of starvation if continually disturbed in foraging areas (Baltic et al., 2005; Hissa et al., 2003). Baines and Richardson (2007) highlight that access restrictions to wintering grounds where large numbers of birds regularly concentrate should be considered.
Flushing distance to disturbance varies depending on the time of year (Baines and Richardson, 2007). In the breeding season, lekking males are more vulnerable to disturbance compared with females on nests (Ruddock and Whitfield 2007; Storch, 2000). Because of the greater risk of disturbance at lek sites and the negative consequences for reproduction, ecotourism at grouse leks needs to be carefully managed (Storch, 2000). Baines and Richardson (2007) recommend that at black grouse breeding areas dogs should be kept on leads from April to August and viewing facilities should be provided for birdwatchers at leks.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Medium evidence
Breeding season (Nesting females) buffer zone = 100-150m
Breeding season (Lekking males) buffer zone = 500-750m
Nonbreeding season buffer zone = 100-150m
Black grouse is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for black grouse when approached by a pedestrian is 101m during the breeding season and up to 100m during the nonbreeding season; FID values up to 100m have been recorded for disturbance from skiers and snow ploughs during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for black grouse during the breeding season is 100-150m for nesting females and 500-750m for lekking males.
Buffer zones to protect black grouse from forestry operations in the UK range from 300 to1000m during the breeding season. A buffer zone of 120m has been recommended to protect black grouse from pedestrian disturbance in Switzerland during the nonbreeding season.
In the UK, black grouse has the potential to be disturbed on breeding grounds as well as at roosting areas and foraging grounds during the nonbreeding season.
Depending on the level of habituation to disturbance, buffer zones of 100-150m for nesting females and 500-750m for lekking males (considered to be the upper disturbance limits estimated by expert opinion (Ruddock and Whitfield, 2007)) are suggested to protect breeding birds from pedestrian disturbance. For forestry activities, buffer zones up to 1000m may be necessary during the breeding season. Buffer zones required to protect nonbreeding birds may be lower, a buffer zone of 100-150m is suggested to protect nonbreeding birds from pedestrian disturbance. For survey work, the monitoring methods presented in Gilbert et al. (1998) should be followed.
Knowledge gaps
Lack of studies measuring AD/FID for pedestrian leisure activity during the breeding season.
Species: Divers and grebes
Red-throated diver, Gavia stellata
Conservation Status
UK: Green List; Schedule 1
European: Least Concern, Annex 1
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 1,250 (1,000-1,550) breeding pairs, 21,500 individuals in winter (Woodward et al., 2020); Scottish population = 935-1,500 pairs, over 2,270 individuals in winter (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a weak increase in breeding birds (+38%) over 12 years.
Winter range expanded by 32% between 1981/84 – 2007/11. Breeding numbers in Scotland increased by 38% between 1994 – 2006. Breeding range increased by 11% between 1968/72 – 2007/11, although a 9% range contraction was recorded between 1968/72 – 2007/11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
FID updates (Díaz et al., 2021; Laursen et al., 2017; Jiang and Møller, 2017) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: FID = 110m (n = 3); Min/Max FID = 100 to 120m (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID = 110m (n = 3) (Jiang and Møller, 2017).
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD = 225m (n = 12 to 13); Min/Max AD (80% opinion range) = 150 to 750m; Min/Max AD (90% opinion range) = 500 to 750m.
Median FID = 125m (n = 14 to 15); Min/Max FID (80% opinion range) = 10 to 750m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season:
Motorised watercraft (motorboat) in nearshore waters off Denmark: Mean FID = 1200m (Laursen et al., 2017).
Non-motorised watercraft (kite surfer) in nearshore waters off Denmark: Mean FID = 1400m (Laursen et al., 2017).
MAD and/or
Buffer zone
Quantitative distances
No buffer zone update published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Safe working distance = 300 to 900m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Ecology and non-quantitative disturbance responses
In the UK, red-throated divers breed only in North and West Scotland and Co. Donegal in Ireland, on freshwater lochs or bog pools in open moorland, blanket bogs or open and wet peatland habitats (Balmer et al., 2013; Snow and Perrins, 1998). The highest breeding densities in Scotland are found in Shetland, parts of Orkney, Caithness, the western fringe of the Highlands and the Outer Hebrides (Balmer et al., 2013). Red-throated divers feed principally on fish; almost all birds at UK breeding sites commute from their freshwater nesting site to feed at sea in nearby shallow coastal areas, so this species is potentially vulnerable to human disturbance at sea as well as on breeding lochs. Human disturbance on and around waterbodies where red-throated divers breed can deteriorate the quality of diver breeding habitat and reduce their breeding success; the use of artificial nesting rafts has been shown to increase breeding success and help mitigate the effects of human disturbance (Nummi et al., 2013; Piper et al., 2002).
In the nonbreeding season, red-throated divers are usually to be found in inshore marine waters along sheltered coasts, only rarely occurring inland on freshwater bodies (Snow and Perrins, 1998). In the UK this species overwinters all around the coast of Britain and Ireland, the highest concentrations are found along the North Sea coasts, in South West Scotland and in South West Ireland (Balmer et al., 2013). This distribution partly agrees with diver distribution recorded during offshore aerial surveys which have revealed large congregations of wintering red-throated divers off South East England, especially in the Greater Thames (Balmer et al., 2013).
Red-throated diver has been assessed as having a very high sensitivity to boat disturbance (Furness et al., 2013); in marine areas this species has been identified as being particularly sensitive to human activities (Dierschke et al., 2016), including through the disturbance effects of ship and helicopter traffic (Mendel et al. 2019; Garthe and Hüppop, 2004; Schwemmer et al., 2011; Furness and Wade, 2012; Bradbury et al., 2014; Dierschke et al., 2016). Marine activity may also increase the number of red-throated diver flights; relative to the other two diver species, red-throated divers are much more likely to take flight in response to disturbance, but they have also been recorded flying more in the absence of disturbance than the other two diver species (Jarrett et al., 2018). Red-throated divers are very likely to take flight in the 200-300m distance band from a passing ferry (Jarrett et al., 2018) and other studies have suggested that this species will fly away from approaching vessels at a distance of at least 1km or more (Garthe and Hüppop, 2004; Schwemmer et al., 2011; Topping and Petersen, 2011). In the German North Sea, Schwemmer et al. (2011) have shown that red-throated divers avoid active shipping lanes. Dierschke et al. (2016) reviewed all available evidence from operational offshore wind farms on the extent of displacement or attraction of seabirds in relation to these structures; a strong avoidance of offshore wind farms was noted for red-throated divers and black-throated divers.
However, as for other diver species, the response to human disturbance may vary between individuals. Within Irish coastal waters during the nonbreeding season, Gittings et al. (2015) found that two out of three red-throated divers flushed at distances of approximately 15m and 100m from a motorised boat, while a third was recorded at a distance of 400 to 500m from the boat, although, as noted by the author, the sample size in this study was very small; flushed birds flew a long way (at least 0.5km and over 1km) from the boat.
Likely sensitivity to disturbance = High
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 500-750m
Nonbreeding season buffer zone = ≤1000m
Red-throated diver is assessed to have a high sensitivity to human disturbance.
Divers have some of the highest AD/FID/MAD values recorded in the bird disturbance response database. Studies measuring AD/FID are limited for red-throated divers, but the maximum AD/FID value recorded for this species is 120m when approached by a pedestrian during the breeding season and 1400m when approached by non-motorised watercraft during the nonbreeding season. Ruddock and Whitfield (2007) suggested that the upper pedestrian disturbance limit for red-throated diver during the breeding season is 500-750m.
Buffer zones range from 300 to 900m for forestry operations during the breeding season.
In the UK, red-throated diver has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds (particularly by boat traffic) at the coast during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 500-750m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect breeding red-throated diver from pedestrian and boating (on breeding lochs) disturbance. For activities with a high potential for visual and audial disturbance (e.g. forestry operations), a buffer zone ≤900m may be necessary. In marine areas during the nonbreeding season, a large buffer zone ≤1km may be necessary to protect foraging and roosting birds from shipping disturbance.
Knowledge gaps
Lack of studies measuring AD/FID during the nonbreeding season. Current research on time budgets of red-throated divers in the nonbreeding season (using time-depth recorders deployed on leg rings on breeding birds) may indicate the extent to which they experience an energy bottleneck during winter and therefore may be vulnerable to impacts on body condition and overwinter survival.
Black-throated diver, Gavia arctica
Conservation Status
UK: Amber List; Schedule 1
European: Least Concern, Annex 1
UK status
Migrant/Resident Breeder, Winter Visitor
UK and Scottish population estimate
UK population = 215 (190-250) breeding pairs, 560 individuals in winter (Woodward et al., 2020); Scottish population = c.200 breeding pairs, 700-800 individuals in winter (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a stable number of breeding birds (+16%) over 12 years.
Believed to have declined during early 20th century due to persecution by anglers and collectors, but has increased since and recovered breeding range that had been lost (Forrester et al., 2012). A 10% breeding range was recorded between 1988/91 – 2007/11 this mirrors national survey results showing an increase from 187 territories in 1994 to 217 territories in 2006 Balmer et al. (2013). Winter range expanded by 51% between 1988/91 – 2007/11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
FID update (Díaz et al., 2021) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: FID = 125m (n = 1) (Díaz et al., 2021).
Motorised watercraft (pedestrian leisure) on a lake in Sweden: Range of mean FID = 189 to 278m (n = 6 to 12); range of median FID = 80 to 310m; Min/Max FID = 0 to 750m (Götmark et al., 1989).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 310 to 400m (n = 10); Min/Max AD (80% opinion range) = 100 to 750m; Min/Max AD (90% opinion range) = 500 to 750m.
Median FID = 225m (n = 10 to 11); Min/Max FID (80% opinion range) = 50 to 500m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone updates published since Ruddock and Whitfield (2007).
Breeding season:
Motorised watercraft (pedestrian leisure) on a lake in Sweden: Buffer zone = >100m around islands where divers are nesting, although an exact figure wasn’t stated (Götmark et al., 1989).
Forestry operations in the UK: Safe working distance = 300 to 900m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in Massachusetts: Safe working distance = 152m, No-cut zone = 30m (Natural Heritage and Endangered Species Program, 2007).
Ecology and non-quantitative disturbance responses
Black-throated diver has a high sensitivity to human disturbance both during the breeding and nonbreeding seasons.
In the UK, black-throated divers breed mainly in the north and west of Scotland (Sutherland and Wester Ross) and the Outer Hebrides (Balmer et al., 2013) in large shallow freshwater lochs or extensive pools with islets and peninsulas (Snow and Perrins, 1998). Loch occupancy is associated with the abundance of small salmonids and complex shorelines (Balmer et al., 2013). In these locations, divers may be disturbed by a range of pedestrian leisure activities, especially activities involving boats. In a study investigating disturbance by fishing activities on black-throated divers, Bundy (1979) found that on larger waterbodies, fishing from the bank did not disturb divers and that adults with chicks kept 50m away from boats, however, on small waterbodies of less than 45ha, divers couldn’t maintain a safe distance and were often absent. Götmark et al. (1989) found that black-throated divers will flush between 189 to 278m from motorised watercraft in areas where they breed. Mudge and Talbot (1993) found that black-throated divers had a high degree of chick mortality in some core areas of their Scottish breeding range between1983-87; almost 80% of nest failure was due to predation and water level changes, but 13% was due to human egg collectors and 5% to desertion following human disturbance. Artificial rafts are increasingly used by black-throated divers to nest upon (Balmer et al., 2013). The use of breeding rafts may moderate effects of fluctuating water levels and human disturbance and have been shown to increase productivity of the Scottish population by 44% (Hancock, 2000).
In the nonbreeding season, black-throated divers generally move to salt water locations around sheltered coasts. Concentrations occur in Cornwall and north west Scotland, and other wintering hotspots occur along the east coast of England and the north coast of Scotland (Balmer et al., 2013). This species can sometimes be seen at inland reservoirs during the nonbreeding season, occasionally frequenting large inland freshwater bodies (Snow and Perrins, 1998). Black-throated divers at sea have been identified as having a high vulnerability to disturbance by boats (Furness et al., 2013) and will often swim or dive in the 200-300m distance band from a passing ferry (Jarrett et al., 2018). In the German North Sea, Schwemmer et al. (2011) have shown that black-throated divers avoid active shipping lanes. It seems likely that this species may avoid areas where marine activity takes place, making data gathering for this species difficult. Black-throated divers are less likely than the smaller red-throated diver to take flight in response to marine activity, instead this species favours a swim or dive response, similar to great northern diver (Jarrett et al., 2018).
Garthe and Hüppop (2004) ranked black-throated diver and red-throated diver as the most sensitive species to offshore wind farm disturbance/displacement impacts. Dierschke et al. (2016) have found that black-throated divers show a significant avoidance of offshore wind farms at more than 2km and that this species can completely disappear around offshore wind farms where formally there was a high density.
Likely sensitivity to disturbance = High
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 500-750m
Nonbreeding season buffer zone = ≤1000m
Black-throated diver is assessed to have a high sensitivity to human disturbance.
Divers have some of the highest AD/FID/MAD values recorded in the bird disturbance response database, although studies measuring AD/FID are limited for black-throated divers., The maximum FID when approached by watercraft during the breeding season is 750m, although the response varies and FID values recorded in other studies are considerably shorter. Ruddock and Whitfield (2007) suggested that the upper pedestrian disturbance limit for black-throated diver during the breeding season is 500-750m. A quantitative measure of FID during the nonbreeding season is not currently available.
Buffer zones of at least 100m have been recommended to protect breeding birds from watercraft disturbance, but out at sea during the nonbreeding season birds will flush from passing boats at a distance of 200-300m. Buffer zones range from 152 to 900m for forestry operations during the breeding season.
In the UK, black-throated diver has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds (particularly by boat traffic) on the coast during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 500-750m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007))is suggested to protect breeding black-throated diver from pedestrian and boating (on breeding lochs) disturbance, but a better understanding of the impact, if any, of disturbance on body condition and survival of black-throated divers would help to inform such decisions. For activities with a high potential for visual and audial disturbance (e.g. forestry operations), a buffer zone ≤900m may be necessary. In marine areas during the nonbreeding season, a large buffer zone ≤1km may be necessary to protect foraging and roosting birds from shipping disturbance.
Knowledge gaps
Lack of studies measuring AD/FID during the nonbreeding season.
Great northern diver, Gavia immer
Conservation Status
UK: Amber List; Schedule 1
European: Least Concern, Annex 1
UK status
Extremely Scarce Breeder, Winter Visitor
UK and Scottish population estimate
UK winter population = 4,400 individuals (Woodward et al., 2020);
Scottish population = 1 possible breeding record, 1,000-3,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
Possibly increasing; distribution increased by 39% between 1981/84 – 2007/11, although apparent gains may be a consequence of improved coverage (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
FID update (Díaz et al., 2021; Jiang and Møller, 2017; Borgmann, 2012; Liley et al., 2010) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in Europe: FID 76.8m (n = 1) (Jiang and Møller, 2017).
Motorised watercraft (pedestrian leisure) on an inland waterbody in Montana: Min/Max FID = 64 to 129m (Kelly, 1992).
Motorised watercraft (pedestrian leisure) on inland waterbodies: Range of mean FID = 10 to 200m (Ruddock and Whitfield, 2007).
Non-motorised watercraft (surveyor canoeing) on an inland waterbody in Wisconsin: Mean FID = 27.8m (n = 30), Min/Max FID = 3 to 90m (Titus and VanDruff, 1981).
Nonbreeding:
Non-motorised watercraft (pedestrian leisure) in a range of habitats and locations: Mean FID = 51m (Borgmann, 2012).
Pedestrian (general) along the shoreline in England: FID = 50m (n = 1) (Liley et al., 2010).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (Borgmann, 2012) published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian (Wisconsin Loon Project): MAD = 67m (Ruddock and Whitfield, 2007).
Pedestrian (Wyoming Bird Conservation Plan): Buffer zone = 165m (Ruddock and Whitfield, 2007).
Motorised watercraft (leisure boat) on an inland waterbody in Montana: MAD = 137m (Kelly, 1992).
Motorised watercraft on lakes in Wisconsin: Buffer zone from the shores of lakes or islands = 150m (Ruddock and Whitfield, 2007).
Human development (Damage Assessment, Remediation and Restoration Program in New England): Buffer zone = 165 to 330m (Ruddock and Whitfield, 2007).
Nonbreeding:
Non-motorised watercraft (pedestrian leisure) in a range of habitats and locations: Buffer zone = 218m (Borgmann, 2012).
Ecology and non-quantitative disturbance responses
Great northern divers are winter visitors to the UK; this species migrates south in winter from arctic breeding grounds. The coastal waters around the UK hold an internationally important wintering population of great northern divers and this species is also occasionally recorded on inland wetland areas and some larger reservoirs (Balmer et al., 2013; Wernham et al., 2002). The largest concentrations of wintering great northern divers are found in the Northern Isles, Outer Hebrides, North West Scotland south to Argyll as well as western and southern Ireland (Balmer et al., 2013). In England, this species is abundant off the Cornish coast (Balmer et al., 2013). Great northern divers feed primarily on fish up to 28cm, but the diet can also include crustaceans, molluscs, annelids, insects and amphibians, depending upon location and season (Snow and Perrins, 1998).
Great northern divers very rarely breed with black-throated divers. A single hybrid pair was recorded in Scotland for several consecutive seasons up to 2008 (Balmer et al., 2013). Birds recorded in the UK during spring are likely to be those migrating north, although small numbers do remain to summer in coastal waters in the north and west (Balmer et al., 2013).
During the breeding season in the high arctic, great northern divers can have a relatively high sensitivity to human disturbance, although the response can vary depending on habituation of individuals and the source of disturbance; disturbance limits of this species may be lower compared with those of red-throated or black-throated diver species (Ruddock and Whitfield, 2007). The majority of studies on breeding great northern divers suggest that they will flush when disturbed on their breeding grounds at a distance of 150 - 300m (Ruddock and Whitfield, 2007), which is generally lower than for black-throated and red-throated divers. Heimberger et al. (1983) found that great northern diver nesting success was greatest when sources of disturbance were beyond 600m. Breeding success has been shown to increase with the use of artificial breeding rafts (Piper et al., 2002).
During the nonbreeding season, great northern divers at sea have been identified as having a high vulnerability to disturbance by boats (Furness et al., 2013, Jarrett et al., 2018); birds are quite likely to swim or dive in the 200-300m distance band from a passing ferry and may also swim (but very rarely fly) out of the path of ferries up to 4km away (Jarrett et al., 2018). In winter, great northern divers spend a high proportion of daylight hours foraging (David C. Jardine, unpublished data) and so it may be difficult to distinguish between behaviours of diving to avoid nearby boats and diving to hunt for food. However, if great northern divers are exposed to an energetic bottleneck in winter, any increase in energy costs caused by disturbance may influence body condition and therefore potentially influence overwinter survival.
FID values vary between individuals. Gittings et al. (2015) found that within Irish coastal waters, great northern divers tolerated a medium sized motorised boat travelling at slow to moderate speeds to within 10 to 20m during the nonbreeding season; great northern divers did not fly away from the boat at this distance, but some individuals did show a dive response at 10 to 20m. Great northern divers also respond to other marine activity, particularly slow vessels/craft (including motorised and non-motorised boats for pleasure and commercial activities) by swimming or diving; in Orkney, they are frequently found in areas where regular marine activity takes place, although rarely recorded close to shore (Jarrett et al., 2018).
In contrast to red-throated and black-throated divers, which tend to avoid areas of human activity such as piers, harbours and ferry terminals, great northern divers can often be watched foraging under piers or in harbours, close to human activity, which suggests that this species, or at least some individuals, are less sensitive to human disturbance than are the smaller diver species (David C. Jardine, pers. comm.).
Likely sensitivity to disturbance = Medium/High
Quantitative information = Medium agreement & Medium evidence
Nonbreeding season buffer zone = 100-350m
Great northern diver is assessed to have a medium to high sensitivity to human disturbance.
The maximum FID value recorded for great northern diver during the breeding season is a mean of 76.8m when approached by a pedestrian and 200m when approached by motorised watercraft. However, as this species does not breed in the UK, quantitative values recorded during the breeding season may not be relevant to disturbance in the UK. During the nonbreeding season, the maximum FID value recorded is 50m when approached by a pedestrian and a mean of 51m when approached by non-motorised watercraft.
A MAD value of 67m and 137m has been recorded for pedestrian and motorised watercraft disturbance respectively during the breeding season. Buffer zones from 150 to 165m have been reported to protect breeding great northern divers from watercraft and pedestrian disturbance, larger buffers up to 330 may be required for disturbance from human development. A buffer zone of 218m has been reported to protect nonbreeding birds from non-motorised watercraft disturbance.
In the UK, great northern diver has the potential to be disturbed (particularly by boat traffic) on foraging and roosting grounds at the coast during the nonbreeding season. A minimum buffer zone of 100-350m is suggested to protect nonbreeding great northern diver from pedestrian disturbance, but a better understanding of the impact, if any, of disturbance on body condition and survival of great northern divers would help to inform such decisions.
Knowledge gaps
Lack of studies measuring AD/FID for a range of disturbance activities, especially pedestrian activity on the beach during the nonbreeding season.
Slavonian grebe, Podiceps auritus
Conservation Status
UK: Red List; Schedule 1
European: Near Threatened, Annex 1
UK status
Resident Breeder, Winter Visitor
UK and Scottish population estimate
UK population = 28 breeding pairs, 995 individuals in winter (Woodward et al., 2020); Scottish winter population = 300-500 individuals (Forrester et al., 2012). Scottish breeding population has declined since Forrester et al. (2012) estimated 30 (30-80) breeding pairs.
UK long-term trend
Eaton et al. (2021) state a strong decrease in breeding birds (-61%) over 25 years.
Breeding numbers have decreased since 1993 (Balmer et al., 2013). Winter range expanded in Britain and Ireland between 1981/84 – 2007/11; part of this increase may stem from improved survey coverage, increases in Scotland may be in response to an increase in the Icelandic breeding population (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
FID update (Liley et al., 2011) published since Ruddock and Whitfield (2007).
Breeding season (Slavonian grebe):
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 75 to 225 (n = 5); Min/Max AD (80% opinion range) = <10 to 300m; Min/Max AD (90% opinion range) = 150 to 300m.
Range of median FID = 30 to 125m (n = 5); Min/Max FID (80% opinion range) = <10 to 150m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Breeding season (great crested grebe, Podiceps cristatus, stand in species for Slavonian grebe):
Surveyor walking in an urban habitat in Finland: FID = 10m (n = 1) (Díaz et al., 2021).
Pedestrian walking/running around breeding lochs in Scotland: Min/Max FID = 8 to 30m (Summers et al., 1994, cited in Bright et al., 2006).
Pedestrian leisure (boats) on breeding lochs in Scotland: Mean FID = 6.4m (n = 7) (Summers et al., 1994, cited in Bright et al., 2006).
Non-motorised watercraft: Min/Max FID = 0 to 100m (Keller, 1989).
Nonbreeding season (Slavonian grebe):
Pedestrian leisure (walking and watercraft) along the shoreline in England: Median AD = 50m (n = 2), FID = 30 (n = 1) (Liley et al., 2011).
Nonbreeding season (great crested grebe):
Non-motorised watercraft (sailing boat) in nearshore waters off Denmark: Mean FID = 90m
Non-motorised watercraft (kite surfer) in nearshore waters off Denmark: Mean FID = 340m (Laursen et al., 2017).
Vehicle (bus) near a treatment plant in Australia: FID = 70m (n = 1) (McLeod et al., 2013).
Pedestrian (general) along the shoreline in England: Median FID = 100m (n = 3); Min/Max FID = 20 to 100m (Liley et al., 2010).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone updates published since Ruddock and Whitfield (2007).
Breeding season (Slavonian grebe):
Forestry operations in the UK: Safe working distance = 150 to 300m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Ecology and non-quantitative disturbance responses
In the UK, Slavonian grebes breed in Scotland where it is a rare breeding bird at the extreme southern end of the species’ Arctic range; breeding is restricted to the eastern Highlands (Balmer et al., 2013). A female Slavonian grebe did attempt to breed with a great crested grebe in the East Midlands between 2006 and 2008 but breeding was not successful (Balmer et al., 2013). This species breeds on a wide variety of lochs including small, shallow fresh, brackish or slightly alkaline waters between 0.5 and 2m deep and between 1-20ha in area with rich floating, submerged and emergent vegetation (Snow and Perrins, 1998).
Breeding Slavonian grebes can be relatively tolerant of human presence and although they are threatened by predation at nests, by flooding and wave damage, human disturbance of nesting birds is not considered to be a threat (Forrester et al., 2007). However, lake selection may be influenced by human disturbance; in particular bank-anglers, whose presence may keep grebes off eggs for extended periods (Thom, 1986; Summers et al., 2011). Summers et al. (2011) note that Slavonian grebe breeding lochs tend to be located hundreds of metres from roads and houses which they suggest is an indication of human disturbance.
In the nonbreeding season, Slavonian grebes move to sheltered coastal inshore waters up to 10-20m in depth including sheltered bays, lagoons and estuaries, joining immigrants from other Arctic breeding areas (Wernham et al., 2002; Snow and Perrins, 1998). Wintering Slavonian grebes occur around most of the Scottish coast; the highest numbers are recorded in the Northern Isles, northwest Scotland, the Moray Firth, the Firth of Forth and Kintyre. In England, this species is also recorded along the coast of Northumberland and from East Anglia to Cornwall (Balmer et al., 2013). Nonbreeding Slavonian grebes on the sea do not normally come ashore. They forage in shallow marine habitats where they could potentially be disturbed by people on the shore, but, in areas where Slavonian grebes occur regularly, there can be considerable human activity. For example, in Argyll, Orkney and Shetland, Slavonian grebes overwinter in areas with frequent ferry and fishing vessel traffic, salmon and mussel farming activity (Argyll Bird Reports volumes 12 to 29, Upton et al., 2018; Jackson, 2018), and these populations appear to be tolerant of these practices.
However, flushing distances of individual birds depends on the extent of habituation and tolerance of disturbance in different areas (Ruddock and Whitfield, 2007). Slavonian grebe is known to have a very high sensitivity to boat disturbance; this species is very likely to respond to a passing ferry at a distance of 200-300m (the third highest response after black-throated and red-throated divers) by flying away (Jarrett et al., 2018). Slavonian grebes can be absent from areas where regular marine activity takes place; in response to marine activity, the evasive flights of Slavonian grebes are longer/further than for other species (Jarrett et al., 2018).
Likely sensitivity to disturbance = Medium
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone = 150-350m
Slavonian grebe is assessed to have a medium sensitivity to human disturbance.
Studies measuring AD/FID are limited for Slavonian grebe, but the maximum AD/FID values estimated by expert opinion are 300m for AD and 150m for FID when approached by a pedestrian during the breeding season. During the nonbreeding season, the maximum FID value recorded is a median value of 50m when approached by a pedestrian. A wider range of FID studies are available for great crested grebe; the maximum FID value recorded for great crested grebe when approached by non-motorised watercraft is 100m during the breeding season and a mean value of 340m during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance limit for Slavonian grebe during the breeding season is 150-300m. Buffer zones range from 150 to 300m for forestry operations during the breeding season.
In the UK, Slavonian grebe has the potential to be disturbed on its breeding grounds, although, due to the scarcity of breeding Slavonian grebes in the UK, human disturbance is more likely on roosting and foraging grounds at the coast during the nonbreeding season. A minimum buffer zone of 150-350m is suggested to protect both breeding and nonbreeding Slavonian grebe from pedestrian disturbance.
Knowledge gaps
Lack of AD/FID studies during the breeding season.
Species: Diurnal raptors
White-tailed eagle, Haliaeetus albicilla
Conservation Status
UK: Amber List; Schedule 1, 1A and A1
European: Least Concern, Birds Directive Annex 1
UK status
Re-introduced Resident Breeder, Accidental
UK and Scottish population estimate
Scottish population only = 122 breeding pairs (Woodward et al., 2020), in winter the number of adults is same as breeding population (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a strong increase in breeding birds (+1,216%) over 25 years.
White-tailed eagle was once widespread in the UK, but this species was driven to extinction by humans early in the 20th century (Balmer et al., 2013). In Scotland there has been a strong increase following re-introductions, starting slowly in the 1970s. There were 30 pairs in 2003 (Forrester et al., 2012). Population models suggest that the population will increase considerably in the coming years, as well as spread over much of Scotland; density-independent predictive models suggest that the white-tailed eagle population could continue to grow to over 200 pairs by 2025 (Sansom et al., 2016). Re-introductions are now taking place in England, where numbers are also likely to increase.
AD/FID
Quantitative disturbance distances
No AD/FID updates published since Ruddock and Whitfield (2007).
Breeding season (white-tailed eagle):
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD = 510m (n = 8); Min/Max AD (80% opinion range) = 150 to 1000m; Min/Max AD (90% opinion range) = 500 to 750m.
Range of median FID = 125 to 225m (n = 10 to 11), Min/Max FID (80% opinion range) = 50 to 1000m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Breeding season (bald eagle, Haliaeetus leucocephalus, stand in species for white-tailed eagle):
Pedestrians walking/running and motorised vehicle (general) in the USA:
Mean FID = 200m, Min/Max FID = 50 to 990m
(Fraser et al., 1985)
Nonbreeding season (bald eagle):
Pedestrian walking/running in North America: Min/Max FID = 183 to 268m
Motorised watercraft in North America: Range of mean FID = 136 to 276m
Non-motorised watercraft in North America: Min/Max FID = 111 to 202m
Fishing boat in North America: Range of mean FID = 127 to 137m.
Bank angler in North America: Mean FID = 201 to 293m
(Stalmaster and Kaiser, 1997)
Aircraft disturbance in North America: Mean FID = 625 to 800m
(Grubb and King, 1991; Fleischner and Weisberg, 1986).
Unknown season (African fish eagle, Haliaeetus vocifer, stand in species for white-tailed eagle):
Surveyor walking in Africa: Mean FID = 68m (n = 2) (Weston et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
Update on buffer zones (SNH, 2015; Kortland et al., 2011; Horváth, 2009; Naylor, 2009) published since Ruddock and Whitfield (2007).
Breeding season (white-tailed eagle):
Forestry operations in Scotland: Buffer zone = 250 to 500m (Kortland et al., 2011).
Forestry operations in Scotland: Safe working distance = 500m (Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 900 to 1100m (Petty, 1998).
Forestry operations (tree felling) in Hungary: Buffer zone = 300 to 400m (Horváth, 2009).
Forestry operations in Finland: Buffer zone = 50 to 500m
Pedestrian walking/running in forest land in Finland: Buffer zone = 500m
Pedestrian camping in forest land in Finland = 1000m
Motorised vehicles (general) in forest land in Finland: Buffer zone = 1000m. (Koivusaari et al., 1988a,b).
Forestry operations in Sweden: Buffer zone = 500m
Industrial development in Sweden: Buffer zone = 2000m
Recommended general buffer zone in Sweden: Buffer zone = 500m
(Ruddock and Whitfield, 2007).
Public viewing platform on the island of Mull in Scotland: Buffer zone = 300m
Public parking area on an island in Scotland: Buffer zone = 600m
(MacLennan and Evans, 2003).
Aircraft disturbance in Scotland: Safe working distance = 500-750m (lateral), 1000m (altitudinal) (SNH, 2015).
Nonbreeding season (white-tailed eagle):
Forestry operations in Scotland: Buffer zone = 0 to 250m (Kortland et al., 2011).
Forestry operations (tree felling) in Hungary: Buffer zone = 100m (Horváth, 2009).
Ecology and non-quantitative disturbance responses
White-tailed eagles are resident breeders in the UK. Reintroduced white-tailed eagles now breed in four key breeding areas in the Western Highlands of Scotland: Outer Hebrides, Wester Ross, Skye and the Small Isles and North Argyll centred on Mull (Balmer et al., 2013). Further white-tailed eagles have been reintroduced to East and Central Scotland (Balmer et al., 2013) and most recently to the Isle of Wight where they are showing some signs of territorial behaviour (Pitches, 2021). These areas are all linked with sea coasts, lochs, rivers and wetlands where fish and other aquatic prey can be caught (Snow and Perrins, 1998). As a predator, scavenger and kleptoparasite, white-tailed eagles have a wide-ranging diet including fish, waterbirds, mammals and carrion (Snow and Perrins, 1998). This species prefers to nest in tall, mature trees, although nesting can take place on cliffs and crags and very occasionally on the ground (Snow and Perrins, 1998). The nest is a large structure, composed of big branches and twigs and often driftwood, juniper and seaweed, which is lined with vegetation; breeding birds are monogamous and often pair for life with pairs reusing the same nest (Snow and Perrins, 1998). Adults generally remain in their territories during the nonbreeding season, whereas immature birds can roam widely; some in Scotland travel inland following highland glens until they reach the east coast (Balmer et al., 2013). White-tailed eagles form communal roosts during the nonbreeding season, although territorial pairs may roost singly at or near nest sites (SNH, 2015).
White-tailed eagles are considered to be sensitive to human disturbance, but the level of sensitivity of individual pairs likely depends on the stage of the breeding cycle as well as exposure to and ability to cope with human presence; in remote areas this species may be scarce and unlikely to be encountered by people, which is likely to increase their sensitivity to disturbance. Some studies have shown that white-tailed eagles are much more approachable and more tolerant of human presence than golden eagles, which makes them particularly vulnerable to persecution (Forrester et al., 2012). Wallgren (2003) suggested that there has been a decreased fear of humans in Finnish white-tailed eagles although there was little evidence of habituation over three decades (1970s, 80s and 90s). During the nonbreeding season in Scotland, Kortland et al. (2011) suggest that forestry operations and activities up to and around white-tailed eagle nests may be carried out with little risk of disturbing white-tailed eagles (unless the eagles are actively nest-building which sometimes happens in December and January), although roost sites should be protected from repeated disturbance. To avoid this, forestry activities or recreational events within 250m of an active roost site should be avoided during the period from two hours before sunset until two hours after sunrise, at any time of year.
However, habituation to disturbance can vary widely across different habitats. In a survey recording white-tailed eagle nests in Croatia, Radović and Mikuska (2009) found that more than 95% of the white-tailed eagle population chose to nest more than 1000m away from the nearest human settlement, regardless of the availability of forests, and that nests were located up to 5,690m away from roads; the busier the road the more likely that some eagles chose to nest a long way from it, although illegal killing, nest robbery and hunting activities which still occur regularly in Croatia are likely to influence white-tailed eagle disturbance distances (Mikuska, 2009). At an onshore wind farm in Norway, Dahl et al. (2012) noted that post-construction, white-tailed eagles tended to vacate their territories within 500m from turbine locations and experienced significantly lower breeding success compared with the same territories before construction. Forrester et al. (2012) consider that human activities such as over-fishing inshore and clearance of woodland beside streams with the resultant loss of fish stocks from freshwater lochs may also impact white-tailed eagle populations. Ruddock and Whitfield (2007) noted that in Europe, forestry guidelines generally advise ‘no-cut’ zones around white-tailed eagle nests between 50 and 300m wide, whereas most North American no-cut zones around bald eagle nests are 400m, although these may be reduced in some situations.
In the UK, Hardey et al. (2013) state that white-tailed eagles should not be disturbed from eyries with eggs or small young unless a licenced surveyor has a specific need to record clutch or brood size; when chicks are eight weeks or more old, disturbance at the nest can cause premature fledging. To minimise the risk of disturbance Hardey et al. (2013) recommended that nesting areas are viewed from distances of 500 to 1000m away (Ruddock & Whitfield, 2007; Whitfield et al., 2008a). Adults may be secretive before laying, and, if disturbed during incubation, they will generally slip quietly off the nest and return once the disturbance is over, although it is recognised that different pairs or sites may have different sensitivities to disturbance.
Likely sensitivity to disturbance = High
Quantitative information = Low agreement & Medium evidence
Breeding season buffer zone = 500-1000m
Nonbreeding season buffer zone = 250-500m
White-tailed eagle is assessed to have a high sensitivity to human disturbance in remote areas, although it is important to note that different pairs or sites may have different sensitivities to disturbance; sensitivity may be lower in areas where eagles are habituated to human presence.
Quantitative studies measuring AD/FID are very limited for white-tailed eagle, but, from studies in the USA, the maximum FID value recorded for bald eagle when approached by a pedestrian is 990m during the breeding season and 268m during the nonbreeding season. The maximum FID value recorded for bald eagle during the nonbreeding season is a mean value of 293m when disturbed by fishing activity on the bank. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for white-tailed eagle during the breeding season is 500 to 1000m, although the authors also state that only one of eight respondents considered disturbance (AD) to occur between 750 to 1000m.
Recommended buffer zones for white-tailed eagle vary widely depending on the source of disturbance. Buffer zones to protect white-tailed eagles from forestry operations in Europe range from 50 to 1100m during the breeding season and 0-250m during the nonbreeding season; the majority of forestry buffer zone recommendations during the breeding season, including those for Scotland, range between 250 and 500m. Buffer zones to protect white-tailed eagles from pedestrian disturbance during the breeding season range from 300 to 1000m and a safe working distance for aircraft in Scotland is considered to be 500-700m (lateral) and 1000m (altitudinal).
In the UK, white-tailed eagle has the potential to be disturbed on breeding grounds as well as at communal roosting areas and foraging grounds during the nonbreeding season; this species is most likely to be disturbed pre- and during egg laying early in the breeding season. Depending on the level of habituation to disturbance, a buffer zone of 500-1000m is suggested to protect nesting white-tailed eagles and a buffer zone of 250-500m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian disturbance. Buffer zones at the lower end of these ranges may be sufficient to protect individuals that have some habituation to human presence.
Knowledge gaps
There are a range of studies providing buffer zones for white-tailed eagle, but studies recording AD/FID are required.
Osprey, Pandion haliaetus
Conservation Status
UK: Amber List, Schedule 1
European: Least Concern, Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 240 breeding pairs (Woodward et al., 2020), almost all in Scotland, but reintroduction to Rutland in 1996 has been followed by increase in that area and a spread to Wales (Balmer et al., 2013). Scottish population = 230 breeding pairs in 2017 (Challis et al., 2020), an increase from 182-200 in 2004 estimated by Forrester et al. (2012).
UK long-term trend
Eaton et al. (2021) state a strong increase in breeding birds (+207%) over 25 years.
Ospreys became virtually extinct as a breeding species in Britain during the 1900s due to human persecution, but since natural recolonisation in the 1950s there has been a steady increase in range and abundance in Scotland and northern England (Balmer et al., 2013). A translocation programme at Rutland Water in 1996 is likely to continue to increase numbers (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
No AD/FID updates published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian (general) in the USA: Mean FID = 50m (Carrier and Melquist, 1976).
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD = 225 (n = 12); Min/Max AD (80% opinion range) = 100 to 750m; Min/Max AD (90% opinion range) = 500 to 750m.
Range of median FID = 175 to 225m (n = 12 to 14); Min/Max FID (80% opinion range) = 50 to 750m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season:
Motorised watercraft (powerboat) in nearshore waters off Florida: Mean FID = 57.91m (n = 58); Min/Max FID = 30 to 140m (Rodgers and Schwikert, 2002).
Motorised watercraft (jet-ski) in nearshore waters off Florida: Mean FID = 49.53m (n = 71); Min/Max FID = 20 to 159m (Rodgers and Schwikert, 2002).
Motorised watercraft (airboat) on a lake in Florida: Mean FID = 103m (n = 18) (Rodgers and Schwikert, 2003).
MAD and/or
Buffer zone
Quantitative distances
Update on buffer zones (SNH, 2015; Naylor, 2009; Craig, 2002; Adams and Scott, 1979) published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Safe working distance = 350 to 1,000m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 500 to 800m (Petty, 1998).
Forestry operations in Ontario: Buffer zone = 200m (Naylor, 2009).
Forestry operations in Arizona: Buffer zone = 100m (Adams and Scott, 1979).
Forestry operations in Canada: Buffer zone = 100 to 800m
Forestry operations in Canada next to water edge: Buffer zone = 70 to 350m
(Ewins, 1997).
Pedestrian (general buffer zone) from Colorado Wildlife guidance: Buffer zone = c.402m (Craig, 2002).
Pedestrian (general buffer zone) in USA: Buffer zone = 400 to 1500m (Richardson and Miller, 1997).
Pedestrian (general): Buffer zone = 201m
Forestry operations in Washington State: No-cut zone = 40 to 61m
Forestry operations in Washington State: Restricted-cut zone = 201 to 335m
Motorised Vehicles in Washington State: Buffer zone = 201m
Campsites in Washington State: Buffer zone = 1000m
Hiking trails in Washington State: Buffer zone = 91m
(Rodrick and Milner, 1991).
Aircraft disturbance in Scotland: Safe working distance = 500-750m (lateral), 500m (altitudinal) (SNH, 2015).
Nonbreeding season:
Nearshore water habitat off Florida:
Motorised watercraft (powerboat): Buffer zone = 149m
Motorised watercraft (jet-ski): Buffer zone = 142m
Motorised and non-motorised watercraft: Buffer zone = 150m
(Rodgers and Schwikert, 2002).
Motorised watercraft (airboat) on a lake in Florida: Buffer zone = 250m (Rodgers and Schwikert, 2003).
Forestry operations in Canada: Buffer zone year-round restriction = 40 to 200m (Ewins, 1997).
Ecology and non-quantitative disturbance responses
Ospreys are summer visitors to the UK. Since breeding began at Loch Garten (Inverness-shire) in Scotland in the 1950s, the British osprey population has spread over much of north-east Scotland; the straths and lowlands of the eastern and central Highlands remain a stronghold, with a further significant population breeding in Tayside and central Scotland (Balmer et al., 2013). The species range expanded over the border into Cumbria and Northumberland between 2001-2010 and, due to a translocation programme, this species now breeds at Rutland Water and in Wales (Balmer et al., 2013). In the UK, ospreys are a tree-nesting species breeding near fresh water, often far inland on lochs, pools and rivers (Snow and Perrins, 1998). Ospreys predominately feed on a range of fish species, which are caught in the talons after a shallow dive of no more than 1m (Snow and Perrins, 1998). This species does not spend the winter in the UK, after the breeding season ospreys travel south to overwinter in sub-Saharan Africa (Snow and Perrins, 1998). Ospreys recorded in November and February are late passage birds or birds returning early respectively (Balmer et al., 2013).
Ospreys are considered to be sensitive to human disturbance, but the level of sensitivity of individual pairs likely depends on the stage of the breeding cycle as well as exposure to and ability to cope with human presence. Ospreys vary in their ability to habituate to human disturbance, the effect of disturbance on nesting ospreys is influenced by the timing of the disturbance event during the breeding season (Swenson, 1979; Levenson and Koplin, 1984). Swenson, 1979, suggested that if ospreys are habituated to human presence before nesting, their continued presence might not be detrimental to nesting success, whereas Levenson and Koplin (1984) found that forestry logging activity can have significant adverse effects on productivity. In Perthshire, Scotland, a pair of ospreys continued to breed normally in 2015 despite the occurrence of a music festival (T In The Park), which took place in the immediate surrounding area in the summer; mitigation measures put in place to protect the ospreys included: changes to the festival site layout, introduction of buffer zones around the nest (maximum buffer zone of 750m) and restrictions on activities including fireworks and lighting, all of which appeared to be successful in preventing disturbance to the birds (RSPB, 2015). A safe working distance for aircraft in Scotland is considered to be 500-750m (lateral) and 500m (altitudinal) (SNH, 2015), however, it has been noted by Network Rail that ospreys nesting alongside a powerline pylon in northern Scotland will behave normally when filmed from a helicopter at a distance of c.900m away; Network Rail now inform their pilots of this distance and use it to minimise disturbance risks (Andrew Stevenson, pers. Comm.).
Ospreys that are unaccustomed to human activities should be protected from disturbance. Rodrick and Milner (1991) recommend that roads are closed between April 1 and September 15 if they are located within 201m of a sensitive pair; the authors also suggest that in wild areas, people should not camp within 1km of occupied nests and hiking trails should not come within 91m of a nest tree. Rodrick and Milner (1991) have also presented a range of management recommendations for osprey that include forestry management around nest trees (see Ruddock and Whitfield, 2007 for review).
Ospreys have adapted well to nesting on a wide range of artificial platforms. In Canada, Ewins (1997) has reported that in some areas up to 70% of occupied osprey nests now occur on artificial support structures. In Alberta, Canada, it is common to see osprey nests on roadside telegraph poles adjacent to major highways, with the birds showing no reaction to high volumes of road traffic. In the UK, it has also been noted that ospreys will successfully breed on artificial platforms, some platforms are in public places (e.g. busy marinas) suggesting that osprey behaviour in the UK can be similar to that recorded in Canada (Andrew Stevenson, pers. Comm.).
In the UK, Hardey et al. (2013) state disturbance around osprey nests should be avoided while breeding birds are displaying, incubating or brooding small young. To minimise the risk of disturbance, Hardey et al. (2013) recommend that nests should be viewed from distances of 500–750 m (Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Likely sensitivity to disturbance = Medium/High
Quantitative information = Low agreement & Medium evidence
Breeding season buffer zone = 350-750m
Osprey is assessed to have a medium to high sensitivity to human disturbance, although different pairs or sites may have a different sensitivity to disturbance; sensitivity may be lower in areas where ospreys are habituated to human presence.
Quantitative studies measuring AD/FID are very limited for osprey, but the highest FID value recorded for this species is a mean of 50m during the breeding season when approached by a pedestrian and a maximum of 159m during the nonbreeding season when approached by a jet-ski. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for osprey during the breeding season is 500 to 750m, although the authors also state that expert opinion of disturbance distances for this species varied widely.
Recommended buffer zones for osprey vary depending on the source of the disturbance. Buffer zones to protect ospreys from forestry operations in the UK range from 350 to 1000m during the breeding season. Buffer zones to protect ospreys from pedestrian disturbance during the breeding season range from 91 to 402m (although campsites may need a wider buffer zone of up to 1000m). A safe working distance for aircraft in Scotland is considered to be between 500 to 900m. In the nonbreeding season, buffer zones between 149 and 250m have been suggested to protect osprey from watercraft disturbance, but as this species does not overwinter in the UK, quantitative values recorded during the nonbreeding season may not be relevant to disturbance in the UK..
In the UK, osprey has the potential to be disturbed at nest sites, especially early on in the breeding season. Depending on the level of habituation to disturbance, a buffer zone of 350-750m is suggested to protect ospreys during the breeding season from pedestrian disturbance. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence.
Knowledge gaps
A wide range of management recommendations exist in the literature suggesting buffer zones for osprey. Empirical studies measuring osprey AD/FID are limited. Further studies, particularly focussing on the AD/FID response to human leisure activities are required for this species.
Golden eagle, Aquila chrysaetos
Conservation Status
UK: Green List, Schedule 1, 1A and A1
European: Least Concern, Annex 1
UK status
Resident Breeder
UK and Scottish population estimate
Scottish population only = 510 breeding pairs (Woodward et al., 2020); c.1,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a stable number of breeding birds (+16%) over 33 years.
Due to human persecution, golden eagles became extinct in England, Wales and Ireland by the middle of the 19th century and the population became increasingly rare and fragmented in Scotland (Forrester et al., 2012). Respite from persecution during the two World Wars together with legal since 1954 allowed this species to survive in remoter Scottish hills and glens and eventually begin to recover (Forrester et al., 2012). In Scotland, Forrester et al. (2012) and Balmer et al. (2013) reported that there were 442 pairs in 2003, with numbers remaining stable from 1982 to 2003. However, (Woodward et al., 2020) found that the population had increased to 510 breeding pairs in Scotland in 2015.
AD/FID
Quantitative disturbance distances
FID updates (Spaul and Heath, 2017; Grubb et al., 2010) published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian walking/running in a shrub-steppe habitat in the USA: Mean FID = 779m (n = 11); Min/Max FID = 200 to 1300m (Spaul and Heath, 2017).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 400 to 625m (n = 15 to 14); Min/Max AD (80% opinion range) = 100 to 1500m; Min/Max AD (90% opinion range) = 750 to 1000m.
Range of median FID = 225 to 400m (n = 25 to 19); Min/Max FID (80% opinion range) = 10 to 1500m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Off-road vehicle in a shrub-steppe habitat in the USA: Mean FID = 414m (n = 121); Min/Max FID = 90 to 1300m (Spaul and Heath, 2017).
Road vehicle in a shrub-steppe habitat in the USA: Mean FID = 553m (n = 107); Min/Max FID = 30 to 1100m (Spaul and Heath, 2017).
Aircraft (helicopter) disturbance across canyonlands in the USA: Mean AD = 400m (n = 8); Mean FID = 200m (n = 8) (Grubb et al., 2010).
Nonbreeding season:
Pedestrian walking/running in farmland habitat in Colorado:
Mean FID = 225m (n = 18); Min/Max FID = 105 to 390m (Holmes et al.,1993).
Motorised vehicle (general) in farmland habitat in Colorado:
Mean FID = 82m (n = 16); Min/Max = 14 to 190m (Holmes et al.,1993).
MAD and/or
Buffer zone
Quantitative distances
Update on buffer zones (SNH, 2015; D’Acunto et al., 2018; Grubb et al., 2010) published since Ruddock and Whitfield (2007).
Breeding season:
Motorised vehicle and pedestrian walking/running (simulated results from a model) across shrub-steppe in the USA: Buffer zone = 600m (D’Acunto et al., 2018).
Pedestrian leisure activity (general) in the USA: Buffer zone = 800m (Ruddock and Whitfield, 2007).
Pedestrian (general) in North America: Buffer zone = 200 to 400m
Noise disturbance in North America: Buffer zone = 800m
Visual/audible disturbance in North America: Buffer zone = 200 to 1600m (Richardson and Miller, 1997).
Forestry operations in the UK: Safe working distance = 750 to 1500m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 900 to 1100m (Petty, 1998).
Forestry operations in Sweden: Buffer zone = 500m (McGrady et al., 2004).
Aircraft disturbance in Scotland: Safe working distance = 1000m (lateral), 500m (altitudinal) (SNH, 2015).
Aircraft (helicopter) disturbance across canyonlands in the USA: Buffer zone = 800m (Grubb et al., 2010).
Nonbreeding season:
Pedestrian walking/running or motorised vehicle across farmland in the USA: Buffer zone = 300m (Holmes et al., 1993).
Ecology and non-quantitative disturbance responses
Golden eagles are scarce resident breeders in the UK. This species is mainly confined to upland areas of the Scottish Highlands north and west of the Highland Boundary Fault and most Hebridean islands throughout the year (Balmer et al., 2013); the Uists, parts of Lewis, Harris and Mull support some of the highest densities in Europe (Forrester et al., 2012). Smaller numbers of golden eagles inhabit the hills and mountains of central and eastern Scotland as well as the Southern Uplands in the Scottish Borders and Dumfries & Galloway (South of Scotland Golden Eagle Project, 2021; Balmer et al., 2013). This species is absent from Orkney and Shetland (Balmer et al., 2013). One lone golden eagle was present in the Lake District for some years after its mate died and, in Ireland, a reintroduction project resulted in three breeding pairs in 2010 (Balmer et al., 2013). Adult golden eagles are highly sedentary and remain in their territories throughout the year, whereas immature birds roam widely within the uplands, although there is little difference in distribution between breeding and nonbreeding seasons (Balmer et al., 2013). Scottish golden eagles show a preference for nesting on cliffs, which may allow greater visibility of their surroundings compared to forest nesting birds in Europe, therefore buffer zones may need to be greater for Scottish breeding golden eagles compared with their European counterparts (McGrady et al., 2004; Ruddock and Whitfield, 2007). Territories may have 1–13 (normally 1–6) alternative nests (Hardey et al., 2013). Golden eagles feed mainly on mammals and birds, but reptiles, occasionally fish and insects, may also be eaten; taken alive or as carrion (Snow and Perrins, 1998). Golden eagles may roost singly or at the nest for territorial birds (SNH, 2015).
Golden eagle is a shy, scarce species which lives in remote areas of Scotland and is sensitive to human disturbance. However, the level of sensitivity of individual pairs likely depends on the stage of the breeding cycle as well as exposure to and ability to cope with human presence. Golden eagles now don’t appear to be affected by pesticides and other pollutants, although this species has probably been negatively affected by the long-term, extractive human use of moorlands by grazing, burning, hunting and forestry (RSPB, 2021a). Persecution still remains a significant problem in the central and eastern Highlands of Scotland where the land is managed for red grouse (Whitfield et al., 2003); in these locations, large areas of suitable golden eagle breeding habitat are unoccupied (Whitfield et al., 2007).
The distance at which golden eagles show no reaction to disturbance varies widely depending on the source of disturbance, individual birds, habitats and the time of the year. Caution should be exercised if applying buffer zones to the UK from studies carried out abroad; for example, many of the FID values and buffer zones listed for golden eagle in this report are from studies carried out in the USA where habituation to disturbance may be greater than it is for some golden eagle individuals present in remote locations in Scotland. Reaction to disturbance can be highly variable between individuals; Spaul and Heath (2017) report that during the breeding season in the USA, some golden eagles do not react to people on foot passing by the nest at 200m, whereas other individuals will react at 1300m. When approached by non-motorised vehicles, the lack of reaction between golden eagles has been found to vary between 400 and 1100m (Spaul and Heath, 2017). Grubb et al. (2010) found that an Apache helicopter in the USA could pass by a golden eagle on a nest at a distance of 400m, whereas other individuals will react to this disturbance at 3000m. Also in the USA, White and Sherrod (1973) found that golden eagles did not flush when a helicopter was 18m from the nest and Boeker (1970) report that golden eagles did not flush when a fixed-wing aircraft was within 60m of a nest site.
In the UK, Hardey et al. (2013) state that golden eagle nests should not be approached in March and early April as this species is particularly sensitive to human disturbance just before and during egg laying. Disturbance behaviour typically involves both adult birds circling together to a great height and often drifting away from the nest; if this behaviour is seen the observer should move away as quickly as possible (Hardey et al., 2013). Observer disturbance at nest sites should also be avoided on particularly wet, hot or cold days as the absence of the adults may result in the chilling or overheating of the eggs or young and disturbance may also cause premature fledging (Hardey et al., 2013).
Likely sensitivity to disturbance = High
Quantitative information = Low agreement & Medium evidence
Breeding season buffer zone = 750-1000m
Nonbreeding season buffer zone = 250-500m
Golden eagle is assessed to have a high sensitivity to human disturbance in remote areas, although this species is scarce and unlikely to be encountered in Scotland. Different pairs or sites may have a different sensitivity to disturbance; sensitivity may be lower in areas where golden eagles have some habituation of human presence.
Quantitative studies measuring AD/FID are very limited for golden eagle in the UK, but the maximum FID value recorded for this species in the USA when approached by a pedestrian is 1300m during the breeding season and 390m during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for golden eagle during the breeding season is 750 to 1000m, although the authors also state that the divergence of opinion on disturbance distance for this species during incubation was greater than that for any other species reviewed.
Recommended buffer zones for golden eagle vary widely depending on the source of disturbance. Buffer zones to protect golden eagles from forestry operations in Europe range from 500 to 1500m during the breeding season. Buffer zones to protect golden eagles from pedestrian disturbance during the breeding season range from 200 to 800m and a safe working distance for aircraft in Scotland is considered to be 1000m (lateral) and 500m (altitudinal).
In the UK, golden eagle has the potential to be disturbed on breeding grounds as well as on roosting and foraging grounds during the nonbreeding season; this species is most likely to be disturbed pre and during egg laying early in the breeding season. Depending on the level of habituation to disturbance, a buffer zone of 750-1000m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect nesting golden eagles and a buffer zone of 250-500m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian disturbance. For activities with a high potential for visual and audial disturbance (e.g. forestry operations), a buffer zone ≥1500m may be necessary.
Knowledge gaps
There is a lack of disturbance distance studies in the UK.
Red kite, Milvus milvus
Conservation Status
UK: Green List, Schedule 1A
European: Least Concern, Annex 1
UK status
Resident/Introduced Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 4,400 breeding pairs (Woodward et al., 2020);
Scottish population = ≥ 273 breeding pairs in 2015 (Challis et al., 2020), 300-350 birds in winter (Forrester et al., 2012).
UK long-term trend
Red kites became extinct outside Wales in the late 19th century due to human persecution. Since the reintroduction of red kites outside of Wales in 1989, the range and abundance in England and Scotland has rapidly increased; the range increased sevenfold between 1988/91 and 2007/11 (Balmer et al., 2013). Reintroduction into Scotland started with the Black Isle in 1989, numbers in north and central Scotland have been doubling about every five years (Forrester et al., 2012).
AD/FID
Quantitative disturbance distances
FID update (Díaz et al., 2021) published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD = 125m (n = 9 to 11); Min/Max AD (80% opinion range) = 10 to 300m; Min/Max AD (90% opinion range) = 150 to 300m.
Range of median FID = 30 to 75m (n = 11); Min/Max FID (80% opinion range) = 10 to 300m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Breeding season (Black kite, Milvus migrans, stand in species for red kite):
Surveyor walking in a rural habitat in Spain: Mean FID = 37.9m (n = 2); Min/Max FID = 35.5 to 40.3m (Díaz et al., 2021).
Unknown season (Black kite):
Surveyor walking in Africa: Mean FID = 26.7m (n = 8) (Weston et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (SNH, 2015) published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Safe working distance = 300 to 600m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 400 to 600m (Petty, 1998).
Aircraft disturbance in Scotland: Safe working distance = 300m (lateral), 500m (altitudinal) (SNH, 2015).
Ecology and non-quantitative disturbance responses
Red kites are resident breeders in the UK. The first reintroduction programme in Scotland took place between 1989 and 1993 when 93 red kites of Swedish origin were reintroduced on the Black Isle. Following this, reintroduction programmes in Scotland have established populations in central Scotland (Stirling area) between 1996 to 2001, Dumfries and Galloway (Castle Douglas area) between 2001 to 2004 and in Aberdeenshire between 2007 to 2009 (Forrester et al., 2012; RSPB, 2018). In England, red kites were introduced into the Chilterns in 1989, by 2011 this population had increased to over 800 pairs and since this time the population has spread to colonise much of central southern England and satellite populations have spread to Wiltshire, Hampshire and Sussex (Balmer et al., 2013). The remnant native Welsh population has also expanded since the early 1990s and now covers most of Wales and parts of Shropshire and Herefordshire (Balmer et al., 2013).
Red kites prefer habitats containing open stands of woodland for nesting and communal roosting in winter (Forrester et al., 2012). This species builds a nest composed of dead twigs usually in trees (rarely on a cliff ledge or crag), and often old buzzard or raven nests will be reused (Snow and Perrins, 1998); in Scotland, most nests are in Scots pine or oak (Forrester et al., 2012). Red kites have a varied diet; they are mainly scavengers although they will also take live prey such as small mammals and birds (Snow and Perrins, 1998). In the UK, red kites are sedentary and do not migrate; in the winter this species may disperse short distances to supplementary feeding grounds, breeding and nonbreeding distributions are similar (Balmer et al., 2013).
Red kite is a species that associates closely with humans and in the past this species flourished in areas of human habitation. Red kite was once a common bird seen over London where they would feed in the city waste dumps, much like black kites do in some Indian cities today (N. Goodship pers. obs). In 1544, William Turner recorded red kites taking bread from the hands of children and fish from women; the Greek poet Homer called them ‘snatchers’ as they had a reputation for stealing hats off people’s heads (see Colwell, 2021 for review). There are also other historical records of red kites stealing herring and fish processing waste from workers on the shores of Loch Fyne (Baxter and Rintoul, 1953), and stealing food from the hands of children in the streets of other UK cities (Raye, 2021).
Today, red kites can be seen foraging over farmland, rough grasslands and heath (Snow and Perrins, 1998) where humans are present. In agricultural areas, this species may associate closely with tractors ploughing the ground in order to take earthworms, farmyards where they scavenge for waste, as well as roads where they scavenge for roadkill (Wildman et al., 1998). Red kites will come close to people when feeding opportunities are provided. For example, this species feeds on bird tables in hundreds of UK gardens where meat is put out for them (Orros and Fellowes, 2014), including in Scotland (Wildman et al., 1998). There are also a number of commercial feeding stations in the UK that encourage large flocks of red kites to come to bait in sites providing public viewing. Katzenberger (2021) concluded that, as the population increased between 1970 and 2020 as a consequence of reduced persecution, red kites in Germany moved closer to human settlements, which suggests a reduction in human avoidance by this species and most likely reflects the change from being persecuted to being protected.
However, despite their apparent tolerance of humans, red kites are still potentially sensitive to disturbance, especially early on during the breeding season when birds are laying and incubating as well as when present at communal roosts. In the UK, Hardey et al. (2013) recommend that searches for nests in woodland should not be carried out between mid-March and mid-April (once kites start to display) as disturbance at this stage of breeding may cause a pair to move several kilometres away; if disturbed whilst nest building (such as tree felling in the nesting wood), a breeding pair may stop nest building and start again with a new nest 500-1000m away. To minimise the risk of disturbance Hardey et al. (2013) recommended that nests are viewed from distances of 150–300m (Ruddock and Whitfield, 2007; Whitfield et al., 2008a) and that no attempt should be made to locate the roosts of breeding red kites, as this causes excessive disturbance.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 150-300m
Nonbreeding season buffer zone = 150-300m
Red kite is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for red kite, but the maximum FID value recorded for black kite is 40.3m when approached by a pedestrian during the breeding season; there are no records of AD/FID values during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for red kite during the breeding season is 150-300m.
Buffer zones to protect red kites from forestry operations in the UK range from 300 to 600m during the breeding season. A safe working distance for aircraft in Scotland is considered to be 300m (lateral) and 500m (altitudinal).
In the UK, red kite is most likely to be disturbed at nest sites early on in the breeding season as well as at communal roosting areas during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 150 to 300m 500m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect both breeding and nonbreeding red kites from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence. For activities with a high potential for visual and audial disturbance (e.g. forestry operations, aircraft), a buffer zone between 300-600m may be necessary. For activities with a high potential for disturbance (e.g onshore wind farms), a buffer zone up to 5km may be necessary.
Knowledge gaps
Lack of AD/FID studies during both the breeding and nonbreeding seasons.
Marsh harrier, Circus aeruginosus
Conservation Status
UK: Amber List; Schedule 1
European: Least Concern; Annex 1
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 590-695 breeding pairs (Woodward et al., 2020); Scottish population <10 breeding pairs between 2003-2015 (Challis et al., 2020), 10-100 birds during spring passage and 10-40 birds during autumn passage (Forrester et al., 2012). There were 10-12 occupied home ranges in Scotland in 2019 which fledged 22 young (Marsh Harrier | Scottish Raptor Monitoring Scheme).
UK long-term trend
Eaton et al. (2021) state a strong increase in breeding birds (+389%) over 25 years.
Marsh harrier temporarily went extinct in the UK at the end of the 19th century, numbers then increased before a crash to just one pair in 1971 (Balmer et al., 2013). Since this time abundance and range have shown a large increase; breeding range doubled between 1988/91 and 2007/11 and the number of breeding females increased by 131% between 1995 and 2005 (Balmer et al., 2013). Woodward et al. (2020) recorded a further increase in UK breeding pairs in 2016.
AD/FID
Quantitative disturbance distances
FID update (Díaz et al., 2021) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Poland: Min/Max FID = 54.6 to 130.1m (n = 2). (Díaz et al., 2021).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 215 to 225m (n = 4); Min/Max AD (80% opinion range) = 10 to 500m; Min/Max AD (90% opinion range) = 300 to 500m.
Range of median FID = 30 to 75m (n = 3), Min/Max FID (80% opinion range) = <10 to 500m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Unknown season (African marsh harrier, Circus ranivorus, stand in species for marsh harrier):
Surveyor walking in Africa: FID = 61m (n = 1) (Weston et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone updates published since Ruddock and Whitfield (2007).
Ecology and non-quantitative disturbance responses
As the name indicates, marsh harriers breed in wetland areas with shallow, standing, fresh or brackish waters surrounded by aquatic vegetation such as standing reeds and reedmace (Snow and Perrins, 1998), which are habitats often associated away from human habitation and disturbance. However, this species can also be found on irrigated fields, rush grassland, fens and peat bogs. Marsh harriers are mainly concentrated in south-eastern areas of England, although there has been some range expansion into northwest England, the Channel Islands, the Isles of Scilly and a few sites in eastern Scotland (Balmer et al., 2013). As a ground nesting species, marsh harriers build a nest in thick marshy vegetation and sometimes in plants growing in shallow water; the nest is composed of a large pile of grass, reeds and small sticks (Snow and Perrins, 1998). This species feeds on a variety of ground and marsh animals, depending on local conditions (Snow and Perrins, 1998).
Marsh harrier is a partial migrant, some British breeders overwinter in Britain while others migrate to southern Europe and northwest Africa or south of the Sahara (Wernham et al., 2002). During the winter in the UK, the highest number of marsh harriers is recorded in a broad coastal band along south-eastern England (Balmer et al., 2013), where they may forage on grassy plains or agricultural areas (Snow and Perrins, 1998), which can bring them into contact with sources of human disturbance, although this species seems able to tolerate and even benefit from humanised environments, such as rice fields (Alves et al., 2014). During the winter, marsh harriers may gather at communal roost sites; gatherings of more than 30 birds have been recorded in north Norfolk, over 20 in Lincolnshire and up to 15 on the Isle of Sheppey in Kent (see Bright et al., 2009 for review).
Marsh harrier is an adaptable and opportunistic species (Wernham et al., 2002); the response to human disturbance may vary between individuals depending on levels of habituation to disturbance. In a Spanish study investigating wetland occupation during the breeding season, García et al. (2015) found that variables affecting occupation included vegetation composition and characteristics, wetland dimensions and distance to other wetlands occupied by marsh harriers; human disturbance (i.e. distance to paths, roads and habitation) was not a factor affecting wetland occupation.
However, other studies have found that marsh harriers are potentially sensitive to human disturbance. Direct persecution, agro-pastoral activities and lead-poisoning may determine wetland occupation in many areas in Europe; human disturbance has been found to affect different aspects of marsh harrier breeding activity such as breeding effort, nest defence or provision of prey for offspring (Fernández and Azkona, 1993; Stanevicius, 2004). Fernández and Azkona (1993) found that a relatively low level of disturbance during the breeding season (such as a quiet pedestrian) can result in reduced parental care and reduced nutrition levels in the young. To minimise the risk of disturbance in the UK, Hardey et al. (2013) recommend that nesting areas are viewed from a distance of 300-500 m, although the reedbed nesting habitat may provide a degree of protection in terms of reducing the visible detection of disturbance by marsh harriers (Ruddock and Whitfield, 2007; Whitfield et al., 2008a). Hardey et al. (2013) discourage searches for roosting birds during the breeding season due to the disturbance that this can cause.
Likely sensitivity to disturbance = Medium
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone = 300-500m
Nonbreeding season buffer zone = 300-500m
Marsh harrier is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for marsh harrier, but the maximum FID value recorded for this species is 130m when approached by a pedestrian during the breeding season; there are no records of AD/FID values during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for marsh harrier during the breeding season is 300-500m, although the authors stated that this estimate was cautionary as survey samples for this species were low.
In the UK, marsh harrier is most likely to be disturbed at nest sites early on in the breeding season as well as at communal roosting areas and potentially foraging grounds during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 300 to 500m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect both breeding and nonbreeding marsh harriers from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence.
Knowledge gaps
Lack of AD/FID studies on marsh harrier, more empirical studies are required.
Hen harrier, Circus cyaneus
Conservation Status
UK: Red List, Schedule 1A
European: Least Concern, Annex 1
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 545 breeding pairs (Woodward et al., 2020);
Scottish population = 460 breeding pairs in 2016 (Challis et al., 2020), 350-450 individuals in winter (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a weak decrease in breeding birds (-29%) over 12 years.
Hen harrier became virtually extinct in mainland Britain by the start of the 20th century, mainly due to persecution by gamekeepers; tiny populations remained on Orkney, the Outer Hebrides and possibly on Kintyre and on Arran (Forrester et al., 2012). Respite from persecution during the two World Wars together with legal protection allowed some recovery time for this species. In the UK plus the Isle of Man, numbers increased from 630 pairs in 1988-89 to 806 pairs in 2004; however, numbers fell again to 662 pairs in 2010 (Balmer et al., 2013). Woodward et al. (2020) reported a further decrease to 545 pairs in 2016. Steep population declines have been reported from Ireland (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
FID update (Booms et al., 2010) published since Ruddock and Whitfield (2007).
Breeding season:
Aircraft (helicopter) in Alaska: Mean FID = 70m (n = 6), Min/Max FID = 30 to 150m (Booms et al., 2010).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 225 to 310m (n = 23 to 24); Min/Max AD (80% opinion range) = <10 to 750m; Min/Max AD (90% opinion range) = 500 to 750m.
Range of median FID = 30 to 225m (n = 27 to 29), Min/Max FID (80% opinion range) = <10 to 750m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (SNH, 2015) published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Safe working distance = 500 to 1000m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 500 to 600m (Petty, 1998).
Operational onshore wind farm in the UK: Distance to nearest nest = 200 to 300m (Madders and Whitfield, 2006).
Aircraft disturbance in Scotland: Safe working distance = 500-750m (lateral), 500m (altitudinal) (SNH, 2015).
Ecology and non-quantitative disturbance responses
Hen harriers are generally scarce resident breeders in the UK. This species usually breeds in heather moorland, farmland and newly afforested uplands throughout Scotland, Ireland and Wales. The highest concentrations of hen harrier are recorded in Orkney, Outer Hebrides (Uists) and Inner Hebrides, parts of the Highlands and locally in the Southern Uplands; smaller numbers are present in northern England, Wales and the Isle of Man (Balmer et al., 2013). Forestry is influencing population trends, but hen harriers usually only inhabit areas with young trees (<15 years); mature tree plantations are not used by this species (Balmer et al., 2013). In Scotland, hen harriers nest on the ground, and the nest, which is a low pile of available vegetation (heather, rushes, grass etc), is constructed in amongst thick marshy vegetation, rarely out in the open (Snow and Perrins, 1998). Male hen harriers may perform a ‘sky-dance’ over breeding territories early in the season (Forrester et al., 2012). Some female hen harriers, and occasionally males, can be aggressive towards people at the nest, even striking an intruder’s head with feet and claws, Hardey et al. (2013) therefore recommend that head protection is used by surveyors approaching a nest site. This species feeds on small birds and rodents, typically by flying low over the ground and pouncing on prey; in the breeding season hen harriers will hunt along transects, following habitat edges (Snow and Perrins, 1998).
The hen harrier is a partial migrant, juveniles especially may disperse in the winter into southern England, Ireland and southwest Europe, but many adults remain in the UK throughout the year (Wernham et al., 2002). It is possible that in late autumn there is a small arrival and passage of wintering birds from Scandinavia, although there is no supporting ringing evidence for this (Forrester et al., 2012; Wernham et al., 2002). The overwintering population, which is probably largely composed of British and Irish breeders, is significantly different from the breeding distribution, with birds wintering in the lowlands, particularly around the coast all around the UK (Balmer et al., 2013). During the winter, hen harriers may gather at communal roost sites; exceptionally large roosts can hold up to 90 birds (in the Isle of Man), but more usually smaller numbers of 3-4 birds roost together, usually in wetlands, but sometimes also on heather moorland, lowland heath, conifer plantations and also in long grass (see Bright et al., 2009 for review).
Hen harriers are sensitive to human disturbance, and persecution in the form of nest destruction has been suggested to limit breeding attempts on grouse moors (Whitfield et al., 2008b). However, some individual hen harriers are able to habituate to human presence; this species can show a wide range of FID responses to different disturbance sources, some seemingly high disturbance activities such as a helicopter or operational wind turbines in the vicinity of nest sites can cause relatively little disturbance, whereas a pedestrian passing by can provoke a response at a relatively large distance (see Ruddock and Whitfield, 2007 for review; Booms et al., 2010; Madders and Whitfield, 2006).
Harriers prefer undisturbed grasslands for nesting (Urquhart, 2011). Tapia et al. (2004) found that hen harriers avoid areas with high levels of human activity such as roads and tracks. Another study found that northern harrier nests did not occur closer than 188m from the nearest building (see U.S. Fish and Wildlife Service, 1991 for review). Hiking trails have also been found to decrease the abundance of wintering harriers in riparian zones (Fletcher et al., 1999). Through habitat modelling, Tapia et al. (2004) suggest that the greatest threats to harrier populations are human infrastructure and human activities such as afforestation and wild-fires that change the habitat conditions.
Hen harriers are especially sensitive to disturbance early on during the breeding season when birds are laying as well as when they are present at roost sites. Hardey et al. (2013) state that if females are disturbed during the laying period, nests containing one or two eggs may be deserted. To minimise the risk of disturbance in the UK, Hardey et al. (2013) recommend that nesting areas are viewed from distances of 500-700m (Ruddock and Whitfield, 2007; Whitfield et al., 2008a) and that care should be taken not to disturb nests in cold or wet weather around the time of hatching or when small young are present. Hardey et al. (2013) also discourage searches for roosting birds during the breeding season due to the disturbance that this can cause.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 300-750m
Nonbreeding season buffer zone = 300-750m
Hen harrier is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for hen harrier, but the maximum FID value recorded for this species is 150m when approached by a helicopter during the breeding season; there are no records of AD/FID values during the nonbreeding season. A non-quantitative study suggests that hen harrier will stay at least 188m away from human habitation. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for hen harrier during the breeding season is 500-750m. Hen harrier will nest at 200 to 300m from an operational wind turbine (Madders and Whitfield 2006) or closer (Ruddock and Whitfield, 2007).
In the UK, hen harrier is most likely to be disturbed at nest sites early on in the breeding season as well as at communal roosting areas and potentially foraging grounds during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 300-750m is suggested to protect both breeding and nonbreeding hen harriers from pedestrian and aircraft disturbance, but habituation to disturbance influences the size of the buffer required and further studies on the impacts of human disturbance are required to help inform such decisions. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to disturbance. For activities with a high potential for visual and audial disturbance (e.g. forestry operations), a larger buffer zone between 500-1000m may be necessary during the breeding period.
Knowledge gaps
There are few studies have directly measured AD/FID for hen harriers, further empirical studies are required particularly focussing on sources of disturbance from human leisure activity.
Common buzzard, Buteo buteo
Conservation Status
UK: Green List
European: Least Concern
UK status
Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 63,000-87,500 breeding pairs (Woodward et al., 2020); Scottish population 15,000-20,000 breeding pairs, 40,000-60,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
Reduced by persecution during late 19th and early 20th century, but this species has subsequently increased with legal protection. Increase has been especially strong in England where the species has spread its range dramatically since 1968-72 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Common buzzard was not included in Ruddock and Whitfield (2007).
Breeding season (common buzzard):
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 49.9 to 88.0m (n = 24); Min/Max FID = 15.3 to 100m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Denmark: FID = 55m (n = 1) (Díaz et al., 2021).
Surveyor walking in a rural habitat in Spain: Range of mean FID = 41.5 to 191.50m (n = 7); Min/Max FID = 34 to 231.2m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Czech Republic: Mean FID = 55.3m (n = 3); Min/Max FID = 40.3 to 70.5m. (Díaz et al., 2021).
Surveyor walking in a rural habitat in France: FID = 55m (n = 1) (Díaz et al., 2021).
Surveyor walking in an urban habitat in France: FID = 25m (n = 1) (Díaz et al., 2021).
Surveyor walking in a rural habitat in Poland: Mean FID = 95.8m (n = 2); Min/Max FID = 30.5 to 161m. (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID 60.3m (n = 26) (Jiang and Møller, 2017).
Surveyor walking over farmland in Denmark: Min/Max FID = 0 to 200m (n = 213) (Sunde et al., 2009).
Breeding season (rough-legged buzzard, Buteo lagopus, stand in species for common buzzard):
Surveyor walking in a rural habitat in Denmark: FID = 20.1m (n = 1) (Díaz et al., 2021).
Surveyor walking in Europe: FID = 20.1m (n = 1) (Jiang and Møller, 2017).
Nonbreeding season (common buzzard):
Surveyor walking in Europe: Mean FID = 54.06m (n = 9) (Møller, 2008a; Møller and Erritzøe, 2010).
Pedestrian (general activity) in Europe: Mean FID = 51.07m (n = 8) (Møller, 2008b).
Nonbreeding season (rough-legged buzzard):
Surveyor walking in Europe: FID = 20.1m (n = 1) (Møller, 2008a).
Pedestrian walking/running in farmland habitat in Colorado: Mean FID = 177m (n = 45); Min/Max FID = 55 to 900m (Holmes et al.,1993).
Motorised vehicle (general) in farmland habitat in Colorado: Mean FID = 71m (n = 62); Min/Max = 9 to 170m.
(Holmes et al.,1993).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Forestry operations in Scotland: Safe working distance = 200m
(Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 300 to 450m (Petty, 1998).
Ecology and non-quantitative disturbance responses
Although buzzards were persecuted in the 18th, 19th and early 20th centuries and were impacted by myxomatosis and organochlorine pesticides in the 1950s-60s, the population has rapidly increased; they are now widespread across the UK and are amongst the most abundant diurnal raptor species in Central Europe, (Balmer et al., 2013; Sunde et al., 2009; Thom, 1986). In the UK this species is most abundant in Wales, southwest and northern England, southern Scotland and the low ground of eastern Scotland, although this species has yet to colonise Shetland (Balmer et al., 2013). Buzzards forage over low vegetation, preferring unimproved pasture. They have a broad diet but rabbits are a key prey species (Balmer et al., 2013). Buzzards rest and nest on trees, rocky crags or cliffs, or rarely on steeply sloping ground. The nest is a substantial structure of branches, twigs, heather and other available material (Snow and Perrins, 1998). This species can occupy a wide variety of habitats that can be relatively undisturbed or densely populated by humans including: forests, woodland edges, agricultural land with isolated trees, hilly slopes, ridges or uplands with some degree of tree cover (Snow and Perrins, 1998; Thom, 1986). Buzzards are largely sedentary in the UK, and breeding and nonbreeding ranges are similar, although the range does expand slightly in the winter owing to the dispersal of immature birds (Balmer et al., 2013).
Due the potential of buzzards to live in close proximity with humans, it is not unexpected to find that this species may be disturbed at shorter distances compared with some other raptors. Studies measuring responses of buzzards to a walking pedestrian found that the FID response was generally lower than 100m with an upper limit of 200m (Díaz et al., 2021; Jiang and Møller, 2017; Sunde et al., 2009; Møller 2008a, b; Møller and Erritzøe, 2010). White and Sherrod (1973) found that rough-legged buzzards did not flush when a helicopter was 18m from the nest, although some caution should be exercised when comparing disturbance distances between common buzzards and rough-legged buzzards, as the latter species is a northerly breeding bird which may be less wary of humans than buzzards in the UK where some persecution still occurs.
Care must be taken to avoid excessive disturbance around buzzard nests during egg laying and early incubation as desertion can occur (Hardey et al., 2013). Santangeli et al. (2012) found that buffer zones greater than 100m around nests in intensively harvested areas in Finland resulted in higher occupancy than when harvesting occurred less than 100m from nests, suggesting that as wide a retention buffer zone as possible should be considered in each case (e.g., an increase in clear-cut distance from 0 to just 50 m more than doubled the occupancy).
Likely sensitivity to disturbance = Low/Medium
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 100-200m
Common buzzard is assessed to have a low to medium sensitivity to human disturbance.
The maximum FID value recorded for buzzard when approached by a pedestrian is 231m during the breeding season and at least 54m (a mean value) during the nonbreeding season, however, the majority of recorded FID values are under 100m during the breeding season. MAD/buffer zones range from 200 to 450m to protect common buzzards from forestry operations during the breeding season in the UK.
In the UK, common buzzard has the potential to be disturbed on breeding grounds as well as at roosting areas and foraging grounds during the nonbreeding season; this species is most likely to be disturbed in breeding territories early in the breeding season. Depending on the level of habituation to disturbance, a buffer zone of 100-200m is suggested to protect both breeding and nonbreeding common buzzards from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence. Forestry operations may require a wider buffer zone up to 450m to avoid disturbance during the breeding period.
Knowledge gaps
A range of FID distances in response to a surveyor walking have been recorded across Europe, but studies investigating other types of human disturbance (e.g. agricultural activities and motorised vehicles) are lacking. Further studies to record AD/FID response to a range of human activities are required, especially during the nonbreeding season.
Honey buzzard, Pernis apivorus
Conservation Status
UK: Amber List; Schedule 1
European: Least Concern; Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = more than 100 territories and at least 60 confirmed breeding pairs in 2020 (Rare Breeding Birds Panel, 2020b);
Scottish population = 50 territories in Scotland in 2020 (Rare Breeding Birds Panel, 2020b). Challis et al. (2020) estimated <10 breeding pairs between 2003-2015. Forrester et al. (2012) estimated possibly up to 50 pairs in 2004 and 2-30 individuals during passage.
UK long-term trend
Eaton et al. (2021) state a weak increase in breeding birds (+57%) over 25 years.
Honey buzzards have spread into upland forests of northern and western Britain, but as this is a very cryptic species, population estimates shouldn’t be too relied upon and there is some uncertainty about trends (Forrester et al., 2007; Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Honey buzzard was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Estonia: FID = 60m (n = 1) (Díaz et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Forestry operations in the UK: Safe working distance = 150 to 600m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 400 to 500m (Petty, 1998).
Ecology and non-quantitative disturbance responses
Honey buzzards are a summer visitor to the UK where they have a patchy distribution during the breeding season. The largest concentrations are along the south coast of England including Dorset through to Kent with other smaller breeding populations in Wales, Norfolk, North Yorkshire and Scotland (northeast, central and southern Scotland) (Balmer et al., 2013). Honey buzzards are superficially similar in appearance to common buzzards, but the former species is a more secretive woodland raptor specialising in mature woodlands with clearings to allow foraging (largely on insects, particularly bees and wasps), as well as mixed landscapes of detached woods, copses, meadows and small wetlands (Balmer et al., 2013; Snow and Perrins, 1998; Thom, 1986). This species breeds on branches or in forks of large trees, usually 10-20m above the ground in nests composed of twigs and green leaves; old carrion or common buzzard nests may be re-used (Snow and Perrins, 1998). Roosts generally occur near to the nest site during the breeding season (Hardey et al., 2013). In Scotland, nest woods can be either plantation forest or an older growth mix of deciduous and conifer trees and usually feature open glades, wooded rides and clear-felled areas (Forrester et al., 2012). Honey buzzards do not overwinter in the UK, after the breeding season birds migrate mainly to west and central regions of Equatorial Africa where they spend the winter in wooded areas (Snow and Perrins, 1998).
Honey buzzard is a cryptic species and their secretive habits sometimes allow them to inhabit woodland areas close to human habitation; this species has been considered to be vulnerable to persecution and/or interference with habitat, especially in the breeding season (Snow and Perrins, 1998). It may be difficult to determine how much honey buzzards are disturbed by human presence as, in contrast to other raptor species (sparrowhawks, goshawks and common buzzards), honey buzzards are usually silent when disturbed by humans at the nest site (Selås, 1997). For honey buzzard nests, Santangeli et al. (2012) reported that buffer zones greater than 100m around nests in intensively harvested areas in Finland resulted in higher occupancy than when harvesting occurred less than 100m from nests, suggesting that as wide a retention buffer zone as possible should be considered in each case (e.g., an increase in clear-cut distance from 0 to just 50 m more than doubled the occupancy).
However, habituation and tolerance of disturbance varies between individual honey buzzards. Some studies have found that this species is more tolerant of human activity than any other raptor species (see Roberts et al., 1999 for review). Roberts et al. (1999) did not find honey buzzards to be particularly sensitive in a study recording locations of nests in forests of central and lowland Britain. Roberts et al. (1999) found that of 48 honey buzzard nesting attempts, 24 (50%) were in trees adjacent to rides, paths or clearings, and a total of 37 (77%) were within 20m; the farthest nest tree was 150m from an access route and only one nest was believed to have failed as a direct result of human disturbance.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 100-200m
Honey buzzard is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for honey buzzard, but the maximum FID value recorded for this species is 60m when approached by a pedestrian during the breeding season. Buffer zone range from 150 to 600m to protect honey buzzards from forestry operations during the breeding season in the UK. In England, breeding honey buzzards are considered to have a high sensitivity to disturbance within 3km and medium sensitivity within an additional 2km around onshore wind farms.
In the UK, honey buzzard has the potential to be disturbed at nest sites early in the breeding season during egg laying and incubation. Depending on the level of habituation to disturbance, a buffer zone of 100-200m is suggested to protect breeding honey buzzards from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence. Forestry operations may require a wider buffer zone up to 600m to avoid disturbance during the breeding period.
Knowledge gaps
A range of FID distances in response to a surveyor walking have been recorded across Europe, but studies investigating other types of human disturbance (e.g. agricultural activities, wind farms and motorised vehicles) are lacking. Further studies to record AD/FID response to a range of human activities are required, especially during the nonbreeding season.
Northern goshawk, Accipiter gentilis
Conservation Status
UK: Green List; Schedule 1
European: Least Concern
UK status
Re-introduced Resident Breeder
UK and Scottish population estimate
UK population = 620+ breeding pairs (Woodward et al., 2020);
Scottish population = 165 breeding pairs in 2017 (Challis et al., 2020), 350-450 individuals in winter (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a strong increase in breeding birds (+206%) over 25 years.
Once widespread in Scotland, but was exterminated in the 1880s as a result of deforestation and persecution (Balmer et al., 2013). Since then, escaped falconry birds or deliberately released birds first bred in Scotland in 1972, and numbers have increased since then, though at highly variable rates in different parts of Scotland (Forrester et al., 2012). Similar increases after release have occurred in Wales and in England (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
AD/FID updates (Díaz et al., 2021; Grubb et al., 2013) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in an urban habitat in Poland: FID = 23.1m (n = 1) (Díaz et al., 2021).
Surveyor walking in a rural habitat in Spain: FID = 140m (n = 1) (Díaz et al., 2021).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 125 to 175m (n = 10); Min/Max AD (80% opinion range) = 10 to 500m; Min/Max AD (90% opinion range) = 300 to 500m.
Range of median FID = 30 to 70m (n = 10), Min/Max FID (80% opinion range) = <10 to 500m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Forestry operations (logging truck noise) in North America:
Min/Max AD = 78 to 167m (Grubb et al., 2013).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (Anonymous, 2012; Naylor, 2009) published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations (blasting) in North America: Buffer zone = 1000m
Forestry operations (vehicle/machine) in North America: Buffer zone = 500m
Forestry operations (helicopter) in North America: Buffer zone = 1000m
(Anonymous, 2012).
Forestry operations in Ontario: Buffer zone = 200m (Naylor, 2009).
Forestry operations in the UK: Safe working distance = 250 to 450m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 375 to 425m (Petty, 1998).
Forestry operations in France/Italy at a disturbed site: 100m (Penteriani and Faivre, 2001).
Pedestrian (general buffer zone) from Colorado Wildlife guidance: Buffer zone = c.800m (Craig, 2002)
Ecology and non-quantitative disturbance responses
Goshawk is a relatively scarce resident species in the UK that is associated with state-owned forests. The highest numbers of breeding birds are present in the Scottish Borders, northeast Scotland and Wales, the latter is a major stronghold of this species, numbers are smaller elsewhere (Balmer et al., 2013). Goshawk breed on branches or in the forks of large trees, often conifers, usually 10-20m above the ground; the nest is composed of twigs and is freshly built each year, either as a completely new structure, or on top of an existing nest (Forrester et al., 2012; Snow and Perrins, 1998). The nests from different years are often clustered within the same tree (Hardey et al., 2013). This species may also occasionally breed in small broad-leaved trees, but they are then more susceptible to disturbance (Wernham et al., 2002). Goshawks are predators with a wide-ranging diet, prey items include birds as small as goldcrests and mammals as large as adult brown hares; pigeons, corvids and thrushes form the main part of the diet during the breeding season (Forrester et al., 2012; Snow and Perrins, 1998). Adults are sedentary and remain in their territories throughout the year, leading to similar patterns of distribution and abundance between seasons, whereas immature birds roam more widely outside key breeding areas (Balmer et al., 2013). Adults recorded outside known breeding areas in the winter may include the occasional continental migrant (Forrester et al., 2012).
Northern goshawk is a shy, scarce species and is sensitive to human presence, especially early in the breeding season; this species is considered to have low to moderate thresholds for new human disturbance (Anonymous, 2012). Hardey et al. (2013) advises that care must be taken to avoid excessive disturbance around goshawk nests during nest building and early incubation as some pairs are prone to desert at this time. Hardey et al. (2013) recommend that surveyors monitor nests from a distance of 300-500 m (Ruddock and Whitfield, 2007; Whitfield et al., 2008a) and the authors state that if disturbed early in the season, breeding goshawks may move up to 2.5 km to another nest site, with some pairs having up to four different nesting areas within their nesting range. In a study in Germany, Saga and Selås (2012) found that when goshawk pairs lost their nests during autumn, winter or early spring by natural causes or human disturbance, the birds often moved 500m or 1km away and constructed new nests elsewhere.
However, disturbance distance for individual goshawks depends on habituation to disturbance. Snow and Perrins (1998) state that this species requires freedom from disturbance but will live close to isolated dwellings or even fringes of towns.
At a disturbed forestry site in Arizona, Grubb et al. (2013) observed that goshawks present on the nest with 15-day old chicks did not appear to respond to logging trucks passing by the nest at 78m and they generally did not respond to passing aircraft, although in most cases aircraft were louder than the logging truck, indicating acclimatization to aircraft noise. Goshawks are generally considered to be much more tolerant to disturbance in urban environments compared with rural ones (Díaz et al., 2021; see Ruddock and Whitfield, 2007 for review).
The type of forest habitat influences goshawk disturbance; in Norway, Saga and Selås (2012) observed that logging did not reduce the proportion of nests used in the second or third breeding season after logging, but that nest reuse was greater in larger areas of mature forest as well as forests with a higher proportion of Norway spruce, which gives better cover than Scots pine and deciduous trees. Santangeli et al. (2012) found that buffer zones greater than 100m around nests in intensively harvested areas in Finland resulted in higher occupancy than when harvesting occurred less than 100m from nests, suggesting that as wide a retention buffer zone as possible should be considered in each case (e.g. an increase in clear-cut distance from 0 to just 50 m more than doubled the occupancy).
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 300-500m
Northern goshawk is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are fairly limited for goshawk in the UK, but the maximum FID value recorded for this species is 140m when approached by a pedestrian and 167m when approached by a logging truck during the breeding season; there are no records of AD/FID values during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for goshawk during the breeding season is 300-500m; the authors noted that this range is generally in line with the published UK and international buffers.
Buffer zones range from 250 to 425m to protect goshawks from forestry operations during the breeding season in the UK; in America buffer zones for forestry operations can go up to 1km.
In the UK, goshawk has the potential to be disturbed on breeding grounds as well as at roosting areas and foraging grounds during the nonbreeding season; this species is most likely to be disturbed in breeding territories early in the breeding season. Depending on the level of habituation to disturbance, a buffer zone of 300-500m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect both breeding and nonbreeding goshawks from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions, especially during the nonbreeding season. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence. Forestry operations may require a wider buffer zone up to 425m to avoid disturbance during the breeding period.
Knowledge gaps
There are a range of studies providing buffer zones for goshawks, but studies recording AD/FID are relatively few. FID empirical studies are required to record habituation levels of individual birds.
Kestrel, Falco tinnunculus
Conservation Status
UK: Amber List
European: Least Concern
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 31,000 breeding pairs (Woodward et al., 2020);
Scottish population = 2,750-5,500 breeding pairs in 2013 (Challis et al., 2020), 15,000-25,000 individuals in winter and 500-1,000 during passage (Forrester et al., 2012).
UK long-term trend
Breeding range in the UK declined by 6% between 1968/72 to 2007/11, UK population declined by 32% between 1995 to 2010, part of an overall decline of 44% since 1970 (Balmer et al., 2013). The decline in the kestrel population is thought to be stronger in Scotland than in England (Forrester et al., 2012), this species declined by 61% in Scotland between 1995-2018 (Harris et al., 2020). Losses have occurred in western Scotland, Wales and sparingly through the midlands and north of Ireland (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Kestrel was not included in Ruddock and Whitfield (2007).
Breeding season (kestrel):
Surveyor walking in a rural habitat in Spain: Range of mean FID = 2.8 to 12m (n = 16), Min/Max FID = 9.6 to 151.7m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Spain: Range of mean FID = 11.8 to 31.6m (n = 9), Min/Max FID = 10.9 to 31.6m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 18 to 48m (n = 6), Min/Max FID = 8.5 to 48m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Hungary: Range of mean FID = 25 to 41.5m (n = 5), Min/Max FID = 12.5 to 91.6m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Czech Republic: Range of mean FID = 31 to 61.3m (n = 6), Min/Max FID = 31 to 61.3m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Poland: Range of mean FID = 19.9 to 117.7m (n = 3), Min/Max FID = 19.9 to 120m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Poland: FID = 40.3m (n = 1) (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID = 32.6m (n = 10) (Jiang and Møller, 2017).
Breeding season (lesser kestrel, Falco naumanni, stand in species for kestrel):
Surveyor walking in Europe: Mean FID = 44.3m (n = 5) (Jiang and Møller, 2017).
Nonbreeding season (kestrel):
Surveyor walking in Europe: Mean FID = 30.08m (n = 6) (Møller, 2008a).
Pedestrian (general activity) in Europe: Mean FID = 18.02m (n = 3) (Møller, 2008b).
Surveyor walking in Europe: Mean FID = 30.94m (n = 9) (Møller and Erritzøe, 2010).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Forestry operations in the UK: Disturbance free zone = 100 to 200m (Petty, 1998).
Ecology and non-quantitative information on disturbance responses
The kestrel is one of the most adaptable, widespread and abundant resident raptor species in the UK. Densities are highest in central and eastern England and southwest Ireland, but this species is scarcer in western Scotland, Wales and southwest England (Balmer et al., 2013). Breeding and nonbreeding ranges are very similar in the UK (Balmer et al., 2013). Kestrels inhabit a wide range of habitats, both in uplands and lowlands. Rural habitats include moorland, heathland, grassland, wetlands, woodlands and coastal areas; kestrels will also inhabit many areas close to human activity including: parklands, airfields, railways, motorways and other grass-verge highways, canal and river banks, as well as within human settlements including cities with open green spaces (Snow and Perrins, 1998). The nesting locations of this species are highly variable and include cavities or forks in trees and on cliffs, buildings, occasionally pylons, and they will readily take to nest boxes when available (Snow and Perrins, 1998). Kestrels will alarm call if disturbed or if responding to other raptors or corvids entering the nest area, they may also be seen displaying over the nesting territory (Hardey et al., 2013). This species is adaptable and opportunistic in its foraging behaviour; the diet is chiefly small mammals (especially voles), although birds, insects and lizards may also be taken depending upon location and season (Snow and Perrins, 1998).
The kestrel is considered a human-tolerant species, they occur in a variety of human-dominated environments including urban, suburban and agricultural habitats and are therefore able to habituate to at least some degree of human presence. However, although recorded FID values are generally lower for kestrel than for some other raptor species (e.g. Díaz et al., 2021), some studies have shown that kestrel breeding success can be impacted by human disturbance (Strasser, 2010). In a study on American kestrels, Strasser and Heath (2013) found that birds nesting in areas with higher levels of vehicle traffic were 9.9 times more likely to fail than birds nesting in lesser disturbed areas (the habitat and clutch initiation dates did not explain the reproductive outcome). In addition, proximity to large, busy roads and developed areas was found to negatively affect kestrel reproduction by causing increased stress hormones that promoted nest abandonment. The authors of the study suggested that their results demonstrated that the presence of kestrels in human-dominated areas does not necessarily indicate a tolerance for human presence and that disturbance may cause physiological stress responses that impact survival.
Negro and Hiraldo (1993) found that the breeding success of lesser kestrels in Spain was positively correlated with the height of their nests and it was suggested that birds selected the highest positions to avoid predation and disturbance (by carnivores or humans). However, response to human disturbance may differ between kestrels and lesser kestrels as the former is usually a solitary nesting species, whereas lesser kestrel is colonial breeder, sometimes breeding in colonies up to 500 pairs (Snow and Perrins, 1998).
Hardey et al. (2013) advises that care must be taken to avoid excessive disturbance around kestrel nests while pairs are displaying and laying as this may cause the birds to move location. Disturbance at kestrel nests should also be avoided when the chicks are three weeks old or more because they are prone to fledge prematurely from this age (Hardey et al., 2013).
The kestrel population is declining in the UK; this may be a consequence of the recovery of the buzzard population (through better protection) which competes with kestrels for small mammalian prey (Forrester et al., 2012). In addition, kestrels may be suffering from predation from the increasing UK population of goshawk and peregrine predators (Forrester et al., 2012). Concern has been raised by NatureScot over excessive kestrel disturbance at a site in northeast Scotland (NatureScot, 2019); the additional potential for stress caused by excessive human disturbance may increasingly have a detrimental impact upon this species.
Likely sensitivity to disturbance = Low/Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone ≤ 50m
Kestrel is assessed to have a low to medium sensitivity to human disturbance.
The maximum FID value recorded for kestrel when approached by a pedestrian is 152m during the breeding season and at least 31m (a mean value) during the nonbreeding season; the majority of recorded FID values are under 50m during the breeding season. Buffer zones range from 100 to 200m to protect kestrels from forestry operations during the breeding season in the UK.
In the UK, kestrel has the potential to be disturbed on breeding grounds as well as at roosting areas and foraging grounds during the nonbreeding season; this species is most sensitive to disturbance early in the breeding season. Depending on the level of habituation to disturbance, a buffer zone of 100-200m is suggested to protect nesting kestrels and a buffer zone of ≤50m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian disturbance. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence.
Knowledge gaps
A range of FID distances in response to a surveyor walking have been recorded across Europe, but studies investigating other types of human disturbance (e.g. agricultural activities and motorised vehicles) are lacking. Further studies to record AD/FID response to a range of human activities are required, especially during the nonbreeding season.
Eurasian hobby, Falco subbuteo
Conservation Status
UK: Green List; Schedule 1
European: Least Concern
UK status
Migrant/Resident Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 2,050 breeding pairs (Woodward et al., 2020), Challis et al. (2020) estimated 632 breeding pairs in UK;
Scottish population is fewer than five breeding pairs, 10-30 individuals during passage (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a weak increase in breeding birds (+48%) over 25 years.
Hobby has undergone a large-scale expansion in range, consolidating their distribution in southern England and spreading north (Balmer et al., 2013). The UK population increased by 16% between 1995 and 2010; between 2008-11 this species was found to occupy four times as many 10 km squares as in 1968-72 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Hobby was not included in Ruddock and Whitfield (2007).
No AD/FID distances available for hobby.
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Forestry operations in the UK: Safe working distance = 180 to 450m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Ecology and non-quantitative disturbance responses
The hobby is a summer visitor to the UK. Hobbies are a fairly rare breeding species in Scotland, but south of a line from the Humber to the Mersey, this is a widespread breeding species (with the exception of west Wales and Cornwall where they remain scarce) (Balmer et al., 2013). Hobbies breed in lowland habitats with open expanses of low vegetation broken up by groups of tall trees or fringed by mature woodlands, warm enough to sustain an abundance of insect prey, principally dragonflies (Balmer et al., 2013; Snow and Perrins, 1998), however, as this species has spread north, lowland farmland areas have been increasingly used for breeding (Messenger and Roome. 2007; Sergio and Bogliani 1999). Hobbies nest on trees between 6 and 32m tall, usually in old carrion crow nests (Snow and Perrins, 1998). This species is relatively common in cultivated landscapes in Europe (Fuller et al., 1985; Bogliani et al., 1994) and they are able to adapt fairly well to intensively managed agroforestry systems (Sergio and Bogliani, 1999; 2000). Hobbies don’t overwinter in the UK, after the breeding season they migrate south to warmer latitudes and spend the winter mainly in southern Africa (Wernham et al., 2002).
Hobbies show some ability to habituate to human disturbance in farmland areas. Messenger and Roome (2007) observed that in a study of breeding hobbies on lowland farmland in Derbyshire, birds were generally unconcerned by the presence of humans inside vehicles near a nest site, but were usually alarmed by humans on foot close to nest sites; three cases of human related nest failures were thought to be due to unintentional disturbance by farmers or others working outside vehicles for extended periods in the immediate vicinity of the nest. Sergio and Bogliani (1999) documented similar tolerance to human disturbance, in Italy some hobby pairs appear to be extremely tolerant of humans inside tractors, some birds have also been observed to continue incubation whilst the ground just underneath the nest is ploughed. Sergio and Bogliani (1999) also reported that the local hobby population in their Italian study area appeared to have adapted fairly well to the intensively managed agroforestry system, with a recorded density and productivity in the range being similar to that reported for other European hobby populations in less intensively cultivated areas.
However, despite some tolerance shown towards human presence, hobbies are possibly still more likely to choose breeding habitats away from human disturbance if suitable habitat is available. In another study investigating hobby nest site selection in Italy, Sergio and Bogliani (2000) observed that hobbies select nesting areas with a higher extent of mature poplar plantations and further away from potential sources of human disturbance; mean distances of nest sites from roads ranged from 1,004 to 1,255m and mean distance to human habitation ranged from 1,024 to 1,546m. Hardey et al. (2013) advises that hobbies are particularly sensitive to disturbance during early incubation and that intensive nest searches are best carried out at a time when young are likely to have hatched.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 200-450m
Eurasian hobby is assessed to have a medium sensitivity to human disturbance, but this is a cautionary assessment due to the lack of available published studies reporting AD/FID values for this species.
Buffer zones range from 180 to 450m to protect hobbies from forestry operations during the breeding season in the UK.
In the UK, hobby has the potential to be disturbed at nest sites early in the breeding season during egg laying and incubation. Depending on the level of habituation to disturbance, a buffer zone of 200-450m is suggested to protect breeding hobbies from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence.
Knowledge gaps
There is little published on the effects of human disturbance on hobbies. Studies are required to measure a range of human disturbance on the AD/FID for this species.
Peregrine falcon, Falco peregrinus
Conservation Status
UK: Green List, Schedule 1
European: Least Concern, Annex 1
UK status
Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 1,750 breeding pairs (Woodward et al., 2020);
Scottish population = 523 (479-592) breeding pairs in 2014 (Challis et al. 2020), 2,000-2,500 individuals during winter (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a stable number of breeding birds (+5%) over 22 years.
Although numbers decreased considerably due to organochlorine pesticides in the 1950s-60s, there has been a large increase in numbers after the pesticide ban; a 200% range expansion is reported between 1968-72 to 2008-11 (Balmer et al., 2013). However, population trends in different parts of the UK vary and populations in some upland areas have declined; in contrast with England, the population estimates for Scotland suggest an overall decline between 2002 to 2014 (Wilson et al.,2018),
AD/FID
Quantitative disturbance distances
No AD/FID updates published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 225 to 310 (n = 24 to 26); Min/Max AD (80% opinion range) = 10 to 750m; Min/Max AD (90% opinion range) = 500 to 750m.
Range of median FID = 125 to 225m (n = 30 to 31); Min/Max FID (80% opinion range) = 10 to 500m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season (Prairie falcon, Falco mexicanus, stand in species for peregrine falcon):
Pedestrian walking/running in farmland habitat in Colorado: Mean FID = 92m (n = 33); Min/Max FID = 24 to 185m (Holmes et al.,1993).
Motorised vehicle (general) in farmland habitat in Colorado: Mean FID = 85m (n = 27); Min/Max = 18 to 200m (Holmes et al.,1993).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (Slankard et al., 2020; SNH, 2015) published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian (general buffer zone) from Colorado Wildlife guidance: Buffer zone = c.802m (Craig, 2002).
Pedestrian leisure (climbing) in North America: Buffer zone = 800m
Pedestrian leisure (general) in North America: Buffer zone = 800 to 1500m
Noise disturbance in North America: Buffer zone = 800m
Pedestrian (general) in North America: Buffer zone = 200 to 1600m
(Richardson and Miller, 1997).
Pedestrian leisure (climbing) in the UK: Buffer zone = 200m (Brambilla et al., 2004).
Pedestrian leisure (climbing; walking/running) in North America: Buffer zone = 400 to 800m (Ellis, 1982).
Forestry operations in the UK: Safe working distance = 600 to 1000m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 400 to 600m (Petty, 1998).
Forestry operations in Poland: Strict buffer zone = 200m, Seasonal buffer zone = 500m (see Bright et al., 2006).
Aircraft disturbance in Scotland: Safe working distance = 500-750m (lateral), 500m (altitudinal) (SNH, 2015).
Aircraft n Europe: Buffer zone = 500m (Fyfe and Olendorff, 1976).
Construction activity at a bridge in the USA: Buffer zone = 46 to 91m (Slankard et al., 2020).
Quarrying activities: Buffer zone = 150 to 600m, depending on habituation and tolerance of the individual to human disturbance (British Columbia Ministry of Forests, Lands and Natural Resource Operations, 2013)
Nonbreeding season:
Pedestrian and vehicle disturbance in farmland habitat in Colorado: Buffer zone = 160m (Holmes et al.,1993).
Quarrying activities: Buffer zone = 50 to 500m, depending on habituation and tolerance of the individual to human disturbance (British Columbia Ministry of Forests, Lands and Natural Resource Operations, 2013)
Ecology and non-quantitative disturbance responses
The peregrine falcon is a resident species in the UK. Peregrine falcons are adaptable and highly mobile, they breed in a wide range of environments including uplands and coastal areas with suitable precipitous cliffs and crags as well as across much of the lowlands where they can breed in quarries or trees and man-made structures (Balmer et al., 2013; Snow and Perrins, 1998). Depending on the location of breeding, the nest can be formed of a slight scrape in earth or old nest debris of nest ledge or a depression on top of an old nest of another species (Snow and Perrins, 1998). Peregrines feed chiefly on birds taken on the wing, usually over open country, but if nesting by the coast, hunting may be carried out almost exclusively over the sea during the breeding season (Snow and Perrins, 1998). Prey recorded in Scotland ranges in size from goldcrest to geese with pigeons and red grouse often eaten (Forrester et al., 2012). In the UK, peregrines are non-migratory and breeding and nonbreeding ranges are similar (Balmer et al., 2013), though many individuals, especially immature birds may wonder extensively in autumn and winter (Snow and Perrins, 1998).
Peregrines vary in their tolerance to human disturbance. Generally, undisturbed habitats are preferred for breeding, but the use of man-made structures for nesting by some individuals can be very wide and varied including: tall buildings, bridges, electricity pylons, power stations, chimneys, gas towers, church towers, quarry machinery, ruins and windowsills in high-rise buildings (Balmer et al., 2013; Forrester et al., 2012; Ruddock and Whitfield, 2007 for review). The tolerance level of individual peregrines is likely to depend on the regularity and type of disturbance individuals are exposed to (Ruddock and Whitfield, 2007). Some individual falcons appear to be unaffected by loud disturbance events in close vicinity to the nest, for example, in Alaska, White and Sherrod (1973) found that peregrines did not flush when a helicopter was 18m from the nest and in Australia, Olsen and Olsen (1980) noted that water skiers can regularly pass within 50m of eyries without having any noticeable effect on behaviour. Hardey et al. (2013) consider that pairs in remote locations may be more sensitive to human activity whereas birds in urban areas, quarries or frequently visited sites may be more tolerant of disturbance. Hardey et al. (2013) also state that if licenced surveyors require to record clutch size, incubating peregrines can be flushed from the eyrie during good weather by loud noises (clapping, shouting), but despite such disturbance, some birds may not leave their eggs until the eyrie is reached. Breeding peregrines have been reported to tolerate large amounts of casual disturbance at high, inaccessible cliffs in the UK (see Bright et al., 2006 for review). Moore et al. (1997) state that in the absence of interference to eyries or their occupants, breeding peregrines will ignore most human disturbance. Olsen and Allen (1997) noted that peregrines can be very tolerant of quarrying activity in close proximity to nest sites; an incubating female on a nest located 15m high in a quarry in Australia was noted to return to her nest within ten minutes of blasting occurring within 100m of her nest, three young later successfully fledged from the nest.
However, despite the apparent tolerance of humans shown by some individuals, peregrines are still potentially sensitive to disturbance, especially early on during the breeding season when birds are laying and incubating; for some pairs human presence around the nest can prevent breeding (e.g. Olsen and Olsen, 1980). Ratcliffe (1984) suggested that peregrines don’t flush in the presence of humans “until at close range” but that disturbance may cause nest failure. In the UK, Hardey et al. (2013) recommend that nesting areas are viewed from distances of 500–750m (Ruddock & Whitfield 2007, Whitfield et al., 2008a) to minimise the risk of disturbance and that visits made to the nest by licenced surveyors to measure and ring chicks should be made before the young are 25 days old because after this disturbance to a nest may cause premature fledging. Ruddock & Whitfield, (2007) state that activities above a nest are more likely to cause disturbance than those below.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 500-750m
Nonbreeding season buffer zone ≤ 200m
Peregrine is generally assessed to have a high sensitivity to human disturbance, although response distances by individual birds can vary widely.
Quantitative studies measuring AD/FID are very limited for peregrine, but the maximum FID value recorded for Prairie falcon in the USA is 185m when approached by a pedestrian and 200m when approached by a motorised vehicle during the nonbreeding season; there are no records of AD/FID values during the breeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for peregrine during the breeding season is 500 to 750m.
Buffer zones to protect peregrines from pedestrian disturbance during the breeding season in North America range from 200m to 1.6km, a 200m buffer zone has been suggested to protect breeding birds from climbing disturbance in the UK. Buffer zones to protect breeding peregrines from forestry operations in the UK range from 200 to 600m. A safe working distance for aircraft in Scotland is considered to be 500-750m (lateral) and 500m (altitudinal).
In the UK, peregrine has the potential to be disturbed on breeding grounds as well as at roosting areas and foraging grounds during the nonbreeding season; this species is most likely to be disturbed in breeding territories early in the breeding season. Depending on the level of habituation to disturbance, a buffer zone of 500-750m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect nesting peregrines and a buffer zone ≤200m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions, especially during the nonbreeding season. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence. Forestry operations may require a wider buffer zone up to 600m to avoid disturbance during the breeding period.
Knowledge gaps
A range of buffer zones exist, but very few studies have measured peregrine AD/FID. Further studies, particularly focussing on the AD/FID response to human leisure activities and quarrying activities in the UK are required for this species.
Merlin, Falco columbarius
Conservation Status
UK: Red List, Schedule 1
European: Vulnerable, Annex 1
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 1,150 breeding pairs (Woodward et al., 2020); Scottish population = 733 breeding pairs in 2008 (Challis et al. 2020), 3,000+ individuals in winter (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a weak increase in breeding birds (+94%) over 25 years.
Numbers were thought to be declining slightly up to 1950,and declining faster after 1950. During 1968-72 the population was estimated at 600-800 pairs (of which 280 pairs in Scotland), but surveys in 1983-84 and 1993-94 suggest an increasing population with about 1,100-1,500 pairs (of which 800 pairs in Scotland) (Forrester et al., 2012).
AD/FID
Quantitative disturbance distances
No AD/FID updates published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 225 to 400m (n = 19 to 22); Min/Max AD (80% opinion range) = <10 to 500m; Min/Max AD (90% opinion range) = 300 to 500m.
Range of median FID = 30 to 225m (n = 28 to 30); Min/Max FID (80% opinion range) = <10 to 500m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season:
Pedestrian walking/running in farmland habitat in Colorado: Mean FID = 76 (n = 14); Min/Max FID = 17 to 180m (Holmes et al., 1993).
Motorised vehicle (cars) in farmland habitat in Colorado: Mean FID = 62 (n = 10); Min/Max FID = 44 to 85m (Holmes et al., 1993).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (Naylor, 2009) published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian walking/running or motorised vehicles in farmland habitat in Colorado: Buffer zone = 125m (Holmes et al., 1993).
Pedestrian activity (general) in North America: Buffer zone = 400m (Becker and Ball, 1983).
Forestry operations in the UK: Safe working distance = 200 to 400m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 200 to 300m (Petty, 1998).
Forestry operations in Ontario: Buffer zone = 200m (Naylor, 2009).
Nonbreeding season:
Pedestrian and vehicle disturbance in farmland habitat in Colorado: Buffer zone = 125m (Holmes et al.,1993).
Ecology and non-quantitative disturbance responses
Merlin is a resident breeder in the UK. This species preferentially breeds in upland moorland areas dominated with heather. Scotland holds more than half of the breeding population, the highest densities in the UK are located on Scottish islands, in the northern and eastern Scottish Highlands, the north Pennines and northwest Ireland (Balmer et al., 2013). Merlin chiefly feed on small birds caught in open country (Snow and Perrins, 1998). Like other falcons, merlin do not build their own nests but reuse those created by other species, usually corvids, or they lay their eggs in a scrape on the ground (Snow and Perrins, 1998). Tree-nesting merlin are likely to have a greater detection capability compared with birds nesting on the ground, although tree nesting merlin may respond to human disturbance at shorter distances (see Ruddock and Whitfield, 2007 for review). Breeding merlin roost on the ground in deep vegetation, in trees or on crags close to the nest site Hardey et al. (2013). In the nonbreeding season, wintering merlin are joined by immigrants from Iceland (Wernham et al., 2002). Merlins are much more widespread in the UK during the nonbreeding season, during the winter they tend to avoid uplands and inhabit lower-lying habitats (Balmer et al., 2013).
Merlin is a species known to tolerate some human disturbance and there are many individuals which nest in urban environments (Konrad, 2004; Haney and White, 1999) where reproductive output can be higher than in rural populations (see Ruddock and Whitfield, 2007 for review). Holmes et al., 1993 discussed that merlin may flush at shorter distances when disturbed on a paved road than when disturbed on gravel roads; the authors discussed that the reason for this may be that merlin perching along paved roads have habituated to the greater traffic volume associated with them, or that individuals with greater tolerance limits to disturbance may be using areas with greater disturbance levels.
However, tolerance of disturbance varies between individuals and merlin are potentially sensitive to disturbance, especially early on during the breeding season when birds are laying and incubating. Newton et al. (1981) suggested that increased human recreational disturbance in the Peak District may prevent this species from achieving former breeding numbers in this area. Holmes et al. (1993) showed that merlin were more likely to flush when approached by a human on foot than they were when approached by a vehicle. Besides pedestrians, other human activities may impact breeding merlin including camping and picnic areas, shooting and fishing activities (see Konrad, 2004 for review). Becker and Ball (1983) discussed that established breeding merlin populations may decline from increased stress and reduced productivity if human disturbance is persistent.
In the UK, Hardey et al. (2013) advise that care must be taken to avoid excessive disturbance around occupied merlin nesting ranges in late March and April, as this may cause the birds to move. To minimise the risk of disturbance Hardey et al. (2013) recommended that nesting areas are viewed from distances of 300–500m (Ruddock & Whitfield, 2007; Whitfield et al., 2008a) and that no attempt should be made to locate the roosts of breeding merlin because of the potential for disturbance. Adult merlin flushed from nests may take a long time to return to a nest after disturbance, during which time the eggs are at risk of chilling; small young may also be dislodged (Hardey et al., 2013).
Likely sensitivity to disturbance = Medium
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone = 300-500m
Nonbreeding season buffer zone ≤ 200m
Merlin is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for merlin, but the maximum FID value recorded for this species in the USA is 180m when approached by a pedestrian and 85m when approached by a motorised vehicle during the nonbreeding season; there are no records of AD/FID values during the breeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for merlin during the breeding season is 300 to 500m.
Buffer zones to protect merlin from pedestrian disturbance during the breeding season in North America range from 125 to 400m. Buffer zones to protect breeding merlin from forestry operations in the UK range from 200 to 400m.
In the UK, merlin has the potential to be disturbed on breeding grounds as well as at roosting areas and foraging grounds during the nonbreeding season; this species is most likely to be disturbed in breeding territories early in the breeding season. Depending on the level of habituation to disturbance, a buffer zone of 300-500m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect nesting merlin and a buffer zone ≤200m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions, especially during the nonbreeding season. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence.
Knowledge gaps
There are only a few published studies measuring merlin AD/FID. Further studies, particularly focussing on the AD/FID response to human leisure activities are required for this species.
Species: Waders
Eurasian oystercatcher, Haematopus ostralegus
Conservation Status
UK: Amber List
European: Vulnerable
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 95,500 breeding pairs, 305,000 individuals in winter (Woodward et al., 2020); Scottish population = 84,500-116,500 breeding pairs, 80,000-120,000 in winter (Forrester et al., 2012).
UK long-term trend
Relatively stable, population declined by 29% in Scotland (causes are unclear) contrasting with a 48% increase in England, gains in Britain are almost exclusively at inland sites, though there are some gains along the south coast of England (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Oystercatcher was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Scotland: Mean FID = 20.2m (n = 9); Min/Max FID = 16 to 22m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Scotland: Mean FID = 24.5m (n = 2); Min/Max FID = 24 to 25m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 35 to 45.1m (n = 9); Min/Max FID = 18 to 40m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Denmark: FID = 28m (n = 1) (Díaz et al., 2021).
Surveyor walking in a rural habitat in Norway: Range of mean FID = 18.6 to 24m (n = 14); Min/Max FID = 15 to 35m (Díaz et al., 2021).
Surveyor walking in an urban habitat in Norway: Mean FID = 28m (n = 3); Min/Max FID = 28 to 28m (Díaz et al., 2021).
Surveyor walking in a coastal lagoon habitat in Italy: Mean FID = 43m (n = 62); Min/Max FID = 15 to 105m (Scarton, 2018a).
Motorised watercraft (motorboat) in a coastal lagoon habitat in Italy: Mean FID = 58.1m (n = 63); Min/Max FID = 31 to 92m (Scarton, 2018a).
Nonbreeding season:
Surveyor walking along shoreline in Scotland: Mean FID of foraging birds = 43.81m (n = 165), Min/Max AD = 18 to 68m; FID was less in areas with more human activity (Azaki and Cresswell, 2021).
Surveyor walking over mudflats in Scotland: Mean FID = 137.61m (n = 22) (Dwyer, 2010).
Surveyor walking along a shoreline in England: Mean FID = 97.3m (n = 147); Min/Max FID = 30 to 228m (Collop et al., 2016).
Surveyor walking in an estuary in England: Mean FID = 41m (n = 48) (Brett, 2012).
Surveyor walking along a shoreline in England: Mean FID = 39m (Carless, 2005).
Surveyor walking on mussel bed in England: Mean FID = 123 (n = 27); Min/Max FID = 90 to 140m (Stillman and Goss-Custard, 2002).
Surveyor walking on mussel bed in England: Range of mean FID = 26 to 48m (n = 83) (Urfi et al., 1996).
Surveyor walking in a coastal lagoon habitat in Italy: Mean FID = 76.7m (n = 17); Min/Max FID = 50 to 122m (Scarton, 2018b).
Surveyor walking in mudflats in Denmark: Mean FID = 119m (n = 172), Min/Max FID = 20 to 400m (Laursen et al., 2005).
Pedestrian walking/running along a shoreline in Northern Ireland: Mean FID = 29m (n = 53) (Fitzpatrick and Bouchez, 1998).
Pedestrian walking/running on grasslands in the Netherlands/Germany: Mean FID = 82m (Smit and Visser, 1993).
Pedestrian walking/running on tidal flats in the Netherlands /Germany: Range of mean FID = 85 to 136m; Min/Max FID = 25 to 300m (Smit and Visser, 1993).
Pedestrian leisure (walking and watercraft) along the shoreline in England: Median AD = 40m (n = 19), Min/Max AD = 20 to 80m; Range of median FID = 32.5 to 50m (n = 118); Min/Max FID = 0 to 200m (Liley et al., 2011).
Pedestrian leisure (unspecified) along the shoreline in England: Min/Max AD = 25 to 150m; Median FID = 46m (n = 129); Min/Max FID = 10 to 200m (Liley et al., 2010).
Pedestrian egg collector in the Netherlands /Germany: Mean FID = 46m (Smit and Visser, 1993).
Cattle disturbance in the Netherlands /Germany: Mean FID = 10m (Smit and Visser, 1993).
Agricultural activities in the Netherlands /Germany: Mean FID = 60m (Smit and Visser, 1993).
Aircraft (fixed-winged aeroplane) in the Netherlands /Germany: Mean FID = 500m (Smit and Visser, 1993).
Motorised vehicle (cars) in the Netherlands /Germany: Mean FID = 106m (Smit and Visser, 1993).
Motorised watercraft (motorboat) in a coastal lagoon habitat in Italy: Mean FID = 74m (n = 10); Min/Max FID = 32 to 115m (Scarton, 2018b).
Non-motorised watercraft (kayak) in nearshore waters off Denmark: Mean FID = 60m (Laursen et al., 2017).
Non-motorised watercraft (wind surfer) in nearshore waters off Denmark: Mean FID = 160m (Laursen et al., 2017).
Non-motorised watercraft (kite surfer) in nearshore waters off Denmark: Mean FID = 130m (Laursen et al., 2017).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Surveyor walking in a coastal lagoon habitat in Italy: Buffer zone = 82m. Conservative buffer zone of 100m is proposed (Scarton, 2018a).
Motorised watercraft in a coastal lagoon habitat in Italy: Buffer zone = 85m. Conservative buffer zone of 100m is proposed (Scarton, 2018a).
Nonbreeding season:
Surveyor walking in a coastal lagoon habitat in Italy: Buffer zone = 121m, but this buffer zone would increase to 270m to protect mixed species winter roosts (Scarton, 2018b).
Motorised watercraft in a coastal lagoon habitat in Italy: Buffer zone = 124m, but this buffer zone would increase to 270m to protect mixed species winter roosts (Scarton, 2018b).
Ecology and non-quantitative disturbance responses
Oystercatcher is a widespread species and breeds on almost all UK coasts (Balmer et al., 2013). High densities of breeding birds are associated with the upland margins in eastern Scotland and northern England, as well as with the Northern Isles (Balmer et al., 2013). This species breeds in a wide range of habitats where there may be contact with humans including coastal saltmarshes, sand and shingle beaches, dunes, cliff-tops with short grass and occasionally rocky shores, as well as inland along the shores of lakes, reservoirs and rivers or on agricultural grass and cereal fields, often some distance from water (Snow and Perrins, 1998), As this species share habitats that are often attractive to humans, oystercatchers are often exposed to human disturbance, including trampling on nests and pursuit of chicks and adults by dogs (Tratalos et al., 2021). Tolerance of human disturbance varies between individual oystercatchers Tjørve and Tjørve, 2010); there are a number of studies showing that human recreational disturbance reduces breeding success (e.g. Stillman and Goss-Custard 2002, Verhulst et al., 2001) and that population density is lower in areas where there are high numbers of people (Tratalos et al., 2021). Virzi (2010) found that human disturbance influenced territory choice in American oystercatchers Haematopus palliates. However, there are cases of oystercatchers nesting in suburban areas (Forrester et al., 2007), for example on flat roofs of buildings, in car parks, and on roundabouts. On the other hand, several studies suggest that oystercatcher is less sensitive to disturbance than other wader species, allowing a closer approach and showing habituation to recreational activity and construction work (see literature review in Woodward et al., 2015); Davidson and Rothwell (1993) consider oystercatcher to be less nervous than other wader species. Oystercatchers can show some behavioural plasticity in the choice of foraging areas (van Dijk, 2014; van de Pol et al., 2009; Safriel 1985) and nest site locations (Briggs, 1984; Heppleston 1972) which may allow some adaption to human presence.
In the nonbreeding season, oystercatcher is chiefly a coastal species, frequenting rocky and estuarine shores with the largest concentrations forming on the major estuaries (Balmer et al., 2013); the presence of humans along the shoreline may impact foraging success (Coleman et al., 2003) although Collop (2016) suggested that oystercatcher may be able to cope with a 10% reduction in time spent feeding caused by daily disturbance events on the Wash. Oystercatchers usually roost on the coast at high tide, although they can also roost communally inland (Goss-Custard, 1981). Disturbance from human activity may disrupt sleep patterns and ultimately have fitness implications for this species (McBlain et al., 2020), although for some roosting flocks, disturbance may only marginally affect daily energy expenditure (Linssen et al., 2019). However, the response of roosting birds to human disturbance is likely to depend on the source of disturbance. In a study in North Wales, McBlain et al. (2020) found that human disturbance (particularly pedestrians exercising dogs) at daytime roost sites led to increased vigilance and reduced sleeping time, while increased boat activity (leisure watercraft and commercial boats) resulted in a reduced duration of vigilance but increased “peek” (eye-blinking) frequency, possibly because boat locations were a more predictable source of disturbance than pedestrians. Burton et al. (1996) suggest that after redevelopment at Hartlepool West Harbour, Cleveland, the numbers of roosting oystercatcher declined, despite the creation of a new island roost, likely because of increased disturbance, particularly from people and boats due to the increased access to the marina.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Robust evidence
Breeding season buffer zone = 50-100m
Nonbreeding season buffer zone = 150-300m
Oystercatcher is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for oystercatcher when approached by a pedestrian is 105m during the breeding season and 400m during the nonbreeding season. For motorised watercraft, mean FID values of 58m and 74m have been recorded during the breeding and nonbreeding seasons respectively; during the nonbreeding season, a range of mean FID values between 60-160m have been recorded for non-motorised watercraft. The highest FID value of 500m was recorded for oystercatcher when approached by an aircraft in the nonbreeding season.
During the breeding season, buffer zones of 82m and 85m have been proposed to protect oystercatchers against pedestrian and motorised watercraft disturbance respectively; a conservative buffer zone of 100m has been suggested. During the nonbreeding season, buffer zones of 121m and 124m have proposed for pedestrian and motorised watercraft disturbance respectively, but for flocks of mixed waders containing more sensitive species (e.g. curlew), a buffer zone of 270m is suggested to protect winter roosts.
In the UK, oystercatcher has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season; tolerance of human disturbance may be lower during the nonbreeding season. A buffer zone of 50-100m is suggested to protect nesting oystercatcher and a buffer zone of 150-300m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian and watercraft disturbance.
Knowledge gaps
More studies to specify habituation to disturbance when recording AD/FID for pedestrian activity on the beach and in watercraft, especially during the breeding season.
Ringed plover, Charadrius hiaticula
Conservation Status
UK: Red List
European: Least Concern
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 5,450 (5,250-5,600) breeding pairs, 42,500 individuals in winter (Woodward et al., 2020); Scottish population = 4,900-6,700 breeding pairs, 23,000-25,000 in winter (Forrester et al., 2012).
UK long-term trend
There has been a 23% range contraction in Ireland and a 5% expansion in Britain since 1968-72; the British breeding population declined by c.37% between 1984-2007 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Ringed plover was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 9.0 to 28.5m (n = 38); Min/Max FID = 9 to 40m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Finland: Mean FID = 20.4m (n = 5); Min/Max FID = 10 to 30m (Díaz et al., 2021).
Pedestrian leisure (unspecified) along the shoreline in England: Min/Max FID = 17 to c.100m (Liley and Sutherland 2007).
Nonbreeding season:
Surveyor walking over mudflats in Scotland: FID = 31.91m (n = 1) (Dwyer, 2010).
Surveyor walking along a shoreline in England: Mean FID = 41.1m (n = 30); Min/Max FID = 20 to 74m (Collop et al., 2016).
Surveyor walking in a coastal lagoon habitat in Italy: Mean FID = 47.7m (n = 18); Min/Max FID = 25 to 76m (Scarton, 2018b).
Surveyor walking in mudflats in Denmark: Mean FID = 42m (n = 59), Min/Max FID = 18 to 100m (Laursen et al., 2005).
Surveyor walking along a shoreline in Africa: Mean FID = 16.1m (n = 16.1), Min/Max FID = 10 to 29m (Mikula et al., 2018).
Surveyor walking in Europe: Mean FID = 22.50m (n = 10) (Møller, 2008a).
Surveyor walking along an inland waterbody in Africa: Range of mean FID = 15.7 to 30.5m (n = 63), Min/Max FID = 9 to 36m (Mikula et al., 2018).
Surveyor walking along a river delta in Africa: Mean FID = 24.0m (n = 6),
Min/Max FID = 13 to 40m (Mikula et al., 2018).
Surveyor walking in Africa: Mean FID = 7.8m (n = 12) (Weston et al., 2021).
Pedestrian walking/running on tidal flats in the Netherlands /Germany: Mean FID = 121m; Min/Max FID = 80 to 162m (Smit and Visser, 1993).
Pedestrian leisure (walking and watercraft) along the shoreline in England: Min/Max FID = 30 to 100 (n = 3) (Liley et al., 2011).
Pedestrian leisure (unspecified) along the shoreline in England: Min/Max AD = 50 to 125m; Min/Max FID = 30 to 100m (Liley et al., 2010).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season:
Surveyor walking in a coastal lagoon habitat in Italy: Buffer zone = 77m, but this buffer zone would increase to 270m to protect mixed species winter roosts (Scarton, 2018b).
Ecology and non-quantitative disturbance responses
Ringed plover has a patchy but widespread and mainly coastal distribution in the UK; breeding birds are notably absent from coastal regions of southwest England, Yorkshire and southwest Wales (Balmer et al., 2013), which is due to the lack of suitable nesting beaches in these areas (Wernham et al., 2002). This species tends to be most numerous and concentrated on wide sandy or shingle tidal beaches, with access to suitable resting or nesting places above the highwater mark (Snow and Perrins, 1998). Inland breeding also occurs in some wetland habitats including along rivers, beside lochs and gravel pits, in the midlands of Ireland and in harvested peat bogs (Balmer et al., 2013). Ringed plover is a ground nesting species, usually in the open, but sometimes sheltered by vegetation, never far from water; the nest is a shallow scrape lined with pebbles and vegetation etc. (Snow and Perrins, 1998).
During the winter, ringed plovers are again mainly restricted to coastal areas around the UK where they inhabit muddy, sandy or pebbly coasts (Balmer et al., 2013). Resident breeders are joined by East Atlantic Flyway populations, some resident birds may remain on their breeding grounds during the winter while others move to new coastal areas; some southern and eastern England birds may also migrate to Ireland and Brittany (Wernham et al., 2002). Ringed plovers feed mainly on terrestrial and coastal invertebrates during the breeding season and principally on marine polychaete worms, crustaceans and molluscs during the nonbreeding season (Snow and Perrins, 1998). This species roosts communally, close to feeding sites along the shoreline, on sandbanks or bare arable fields, and in low vegetation (JNCC, 2012).
Ringed plovers are considered to be sensitive to disturbance particularly during the breeding season (see Conway et al., 2008 for review). As ringed plovers predominately breed on sand and shingle beaches which are also attractive to people, they are often exposed to human disturbance, including trampling on nests and pursuit of chicks and adults by dogs (Tratalos et al., 2021). Like other species of plover, if disturbed, ringed plovers will perform a distraction display to lure attention away from chicks or a nest site by running along the ground in a huddled “crouch-run” position, flicking wings, displaying one side of the body and giving an impression of an “exhausted bird” (Williamson, 1947). As this species will often creep along that ground from a disturbance source in this manner, rather than fly away, the estimation of FID for this species can be problematic.
Previous studies have shown that, particularly on the coast, recreational disturbance may affect the distribution, numbers and breeding success of this species (Tratalos et al., 2021, Liley and Sutherland 2007; Tratalos et al., 2005, Brown and Grice, 2005; Pienkowski, 1984). On the eastern shore of the Wash (Norfolk), Liley and Sutherland (2007) found that ringed plovers avoided areas of high disturbance caused by human recreational activity on the beach; a population model suggested that if nests were protected from humans (e.g. by fencing) the ringed plover size would increase by 8% and a complete absence of human disturbance would cause a population increase of 85%. Prater (1976) assessed that disturbance may have altered the habitat choice of ringed plovers in southeast England and on Lindisfarne, Pienkowski, (1984) found that ringed plovers abandoned territories without nesting by mid-May, which appeared to be associated with an increase in the use of the shore by humans at that time of year.
Likely sensitivity to disturbance = High
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 100-300m
Ringed plover is assessed to have a high sensitivity to human disturbance.
The maximum FID value recorded for ringed plover when approached by a pedestrian is 100m during the breeding season and 162m during the nonbreeding season. However, as this species runs rather than flies away when disturbed, FID values are difficult to estimate. During the nonbreeding season, a buffer zone of 77m has been proposed to protect ringed plover against pedestrian disturbance, but for flocks of mixed waders containing more sensitive species (e.g. curlew), a buffer zone of 270m is suggested to protect winter roosts.
In the UK, ringed plover has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season; tolerance of human disturbance may be lower during the nonbreeding season. A buffer zone of 100-200m is suggested to protect nesting ringed plover and a buffer zone of 100-300m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
Lack of studies recording AD/FID during the breeding season. More studies to specify habituation to disturbance when recording AD/FID for pedestrian activity on the beach and in watercraft, especially during the breeding season.
Grey plover, Pluvialis squatarola
Conservation Status
UK: Amber List
European: Least Concern
UK status
Passage/Winter Visitor
UK and Scottish population estimate
UK winter population = 33,500 individuals (Woodward et al., 2020); Scottish population = 1,700-2,800 individuals in winter, 500-2,000 individuals in Spring passage, 5,000-10,000 individuals in Autumn passage (Forrester et al., 2012).
UK long-term trend
Wintering numbers have gradually declined since the mid-1990s, they were 15% lower in 2008-09 compared with 1988-89 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Grey plover was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: Min/Max FID = 36 to 66m (n = 2) (Díaz et al., 2021).
Nonbreeding season:
Surveyor walking along a shoreline in England: Mean FID = 132.3m (n = 55); Min/Max FID = 35 to 251m (Collop et al., 2016).
Surveyor walking in a coastal lagoon habitat in Italy: Mean FID = 77.1m (n = 24); Min/Max FID = 43 to 205m (Scarton, 2018b).
Surveyor walking in a shorebird habitat in Australia: FID = 44m (n = 1) (Glover et al., 2011).
Surveyor walking along a shoreline in Africa: FID = 37m (n = 1) (Mikula et al., 2018).
Surveyor walking along a river delta in Africa: Mean FID = 41.1m (n = 8),
Min/Max FID = 32 to 53m (Mikula et al., 2018).
Surveyor walking in Africa: Mean FID = 38.2m (n = 7) (Weston et al., 2021).
Surveyor walking in Sir Lanka: FID = 33 (n = 1) (Gnanapragasam et al., 2021).
Pedestrian leisure (unspecified) along the shoreline in England: Min/Max AD = 75 to 125m; Median FID = 75m (n = 10); Min/Max FID = 30 to 125m (Liley et al., 2010).
Surveyor walking in mudflats in Denmark: Mean FID = 132m (n = 80), Min/Max FID = 42 to 400m (Laursen et al., 2005).
Pedestrian walking/running on tidal flats in the Netherlands /Germany: Mean FID = 124m; Min/Max FID = 106 to 142m (Smit and Visser, 1993).
Motorised watercraft (motorboat) in a coastal lagoon habitat in Italy: Mean FID = 75.8m (n = 16); Min/Max FID = 46 to 167m (Scarton, 2018b).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season:
Surveyor walking along a shoreline in Africa: Mean MAD = 47m (n = 9) (Boer and Longamane, 1996).
Surveyor walking in a coastal lagoon habitat in Italy: Buffer zone = 148m, but this buffer zone would increase to 270m to protect mixed species winter roosts (Scarton, 2018b).
Motorised watercraft (motorboat) in a coastal lagoon habitat in Italy: Buffer zone = 139m, but this buffer zone would increase to 270m to protect mixed species winter roosts (Scarton, 2018b).
Ecology and non-quantitative disturbance responses
Grey plovers are winter visitors and passage migrants to the UK; this species breeds in Russia and the Canadian high Arctic. Wintering and passage birds are restricted to coastal areas all around the around the UK coastline mostly on areas with intertidal mud and sandflats (Balmer et al., 2013). In Scotland, some of the largest numbers are to be found on the Eden Estuary, Firth of Forth, Solway, Orkney, Outer Hebrides, Tay and Tyninghame estuaries. During migration this species may also be found inland on lakes, pools or grasslands. Grey plover is usually a solitary species or occurs in small flocks while foraging; food is chiefly marine polychaete worms, molluscs and crustaceans during the nonbreeding season (Snow and Perrins, 1998) and like most plovers, grey plovers tend to run and then suddenly stop to feed. Grey plovers form large flocks at communal roosts, often with other waders in sandy areas, such as on unvegetated sandbanks or sand-spits on sheltered beaches or other sheltered environments such as estuaries or lagoons (Snow and Perrins, 1998), therefore there is the potential to disturb this species on foraging and roosting grounds.
Grey plover was among the species noted to be most sensitive to disturbance by walkers and dogs on the Welsh Dee Estuary (see Woodward et al., 2015 for review). Kirby et al. (1993) noted that once grey plover had been disturbed (particularly by walkers and dogs), they were most likely to leave the estuary altogether. Similarly, Ross and Liley (2014) found that grey plovers in the Humber estuary were also among the wader species exhibiting the highest proportion of major flight responses to human recreational disturbance. However, Collop (2016) suggested that, along with curlew, oystercatcher and bar-tailed godwit, grey plover may be able to cope with a 10% reduction in time spent feeding caused by daily disturbance events on the Wash.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Medium evidence
Nonbreeding season buffer zone = 150-300m
Grey plover is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for grey plover when approached by a pedestrian is 66m during the breeding season and 400m during the nonbreeding season. However, as some plovers tend to run rather than fly initially, FID values may be difficult to estimate. As grey plover does not breed in the UK, quantitative values recorded during the breeding season may not be relevant to disturbance in the UK. During the nonbreeding season, buffer zones of 148m and 139m have been proposed to protect grey plover against pedestrian and motorised watercraft disturbance respectively, but for flocks of mixed waders containing more sensitive species (e.g. curlew), a buffer zone of 270m is suggested to protect winter roosts.
In the UK, grey plover has the potential to be disturbed on foraging and roosting grounds during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 150-300m is suggested to protect nonbreeding grey plover from pedestrian and watercraft disturbance.
Knowledge gaps
More studies to specify habituation to disturbance when recording AD/FID for pedestrian activity on the beach and in watercraft during the nonbreeding season.
Golden plover, Pluvialis apricaria
Conservation Status
UK: Green List
European: Least Concern, Annex 1
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 32,500-50,500 breeding pairs, 410,00 individuals in winter (Woodward et al., 2020); Scottish population = 15,000 breeding pairs, 10,000-30,000 individuals in spring passage, 20,000-60,000 in autumn passage, 25,000-35,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
Decrease. Half of the Irish range and one fifth of the British range have been lost over the last 40 years, mirroring the 13% UK population decline (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Golden plover was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: FID = 47m (n = 1) (Díaz et al., 2021).
Surveyor walking in a rural habitat in Finland: Range of mean FID = 18.0 to 43.3m (n = 7); Min/Max FID = 18 to 48m (Díaz et al., 2021).
Surveyor walking in moorland habitat in England: Range of median FID = 191 to 227m (Finney et al., 2005).
Surveyor walking over moorland in Norway: Min/Max FID = 0 to >100m (n = 46) (Byrkjedal, 1987).
Pedestrian walking/running on moorland in England: Min/Max FID = 1 to 200m (n = 96) (Yalden and Yalden, 1990).
Pedestrian walking/running on moorland in England: Mean AD = 187m (n = 333); Min/Max AD = 38 to 491m (Yalden and Yalden, 1989).
Surveyor walking in Scotland: Min/Max AD = 100-300m; Min/Max FID = 50-150m (Andy Douse, pers. obs.).
Nonbreeding season:
Surveyor walking over mudflats in Scotland: Mean FID = 280.9m (n = 2) (Dwyer, 2010).
Surveyor walking in mudflats in Denmark: Mean FID = 143m (n = 38), Min/Max FID = 45 to 450m (Laursen et al., 2005).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Surveyor walking in moorland habitat in England: Mean MAD = 50 to 200m (Finney et al., 2005; Pearce-Higgins et al., 2007).
Pedestrian walking/running on moorland in England: MAD = 200m (Yalden and Yalden, 1990; Yalden and Yalden, 1989).
Ecology and non-quantitative disturbance responses
Golden plover breeds in highland areas and upland bogs, moors and swampy heaths with high abundances of Sphagnum moss and heather. In Scotland, the highest breeding densities occur on the Outer Hebrides, Shetland, the Flow Country of Caithness and Sutherland and in England. High breeding densities occur in the Pennines; breeding densities are low in Ireland and Wales (Balmer et al., 2013). During the breeding season golden plover is a strongly territorial species around the nest site and males perform display flights particularly during early pair formation (Snow and Perrins, 1998; Ratchliffe, 1976), but this behaviour declines during egg laying and individuals can be secretive during the early breeding phase and may not respond to human intrusion (Yalden and Yalden, 1989). Golden plover is a ground nesting species; the nest is a shallow scrape in amongst short vegetation or between stones and is lined with vegetation (Snow and Perrins, 1998).
During the nonbreeding season, golden plover has a widespread distribution around the UK’s lowland fields (Balmer et al., 2013), often in the company of lapwings (Gillings and Fuller, 1999). Resident golden plover in the UK tend to move short distances to their wintering grounds, the majority remain in the UK and are joined by migrants mainly from Iceland (Wernham et al., 2002). Golden plovers are omnivorous, feeding mainly on terrestrial invertebrates (principally beetles and earthworms) but will also feed on some plant material including berries, seeds and grasses (Snow and Perrins, 1998). This species prefers to roost on ploughed arable land and damp grassland, but will use tidal flats, rocky shores and saltmarshes in intertidal areas (JNCC, 2012; Forrester et al., 2012).
Golden plovers are sensitive to human disturbance and numbers are known to be lower in areas of high disturbance (Finney et al., 2005; Pearce-Higgins et al., 2007; Yalden and Yalden, 1989). Some golden plovers will run from their eggs if disturbed, but flight is much more usual (Ratchliffe, 1976). During the breeding season, response to disturbance varies between individual golden plovers depending on a number of factors, including habituation to disturbance, alertness, the vulnerability of the chicks, how conspicuous the disturbance is (e.g. a walker appearing against a skyline may cause more disturbance than a walker hidden in a valley) and the predictability of the source of disturbance (Finney et al., 2005; Yalden and Yalden, 1989). As well as the nature of the breeding grounds, response to human disturbance also depends on whether nesting plovers tend to be “sitters” or “fliers” at the nest; the majority of individuals will fly direct from their nests as a human comes within sight, however, in certain areas or under certain conditions or at certain times, nearly all the birds sit close and flush only if the intruder chances to walk within about 3-10 m of the nest (Ratchliffe, 1976).
Yalden and Yalden (1989; 1990) found that breeding golden plovers are most likely to be disturbed by people walking across moorland if they are within 200m of a nest. Finney et al. (2005) also found that golden plovers avoided pedestrian disturbance across the Pennine Way, however, when this source of disturbance was made more predictable through the resurfacing of the public footpath, golden plovers reduced their avoidance distance of the footpath from 200 to 50m. Pearce-Higgins et al. (2007) discussed that high levels of disturbance can impact golden plover habitat usage, but only in limited circumstances where visitor pressure is very high (greater than at least 30 visitors per weekend day); with the provision of well-surfaced paths, the authors considered that access to large numbers of visitors can be permitted without reducing breeding success. Ratchliffe (1976) suggested that recreational pressures were unlikely to have much effect on breeding golden plover unless the source of disturbance was intense.
Pearce-Higgins et al. (2009) recorded a reduced occurrence of golden plovers within 200m of turbines across 12 upland wind farms. However, Fielding and Haworth (2010) and Douglas et al. (2011) suggest that under some circumstances, golden plovers may be more tolerant of wind farm infrastructure. At Farr wind farm, Fielding and Haworth (2010) showed that the median distance of 16 golden plover nests to the nearest turbine was 168.8m, with nine nests being less than 200m and three less than 100 m from the nearest turbine. At Beinn Tharsuinn wind farm, Douglas et al. (2011) found that the distribution of breeding golden plovers appeared to be unaffected by proximity to turbines or tracks, with no evidence for this lack of association changing through time.
Disturbance studies on golden plover are more limited during the nonbreeding season although flocks can be disturbed on foraging and roosting grounds; Ross and Liley (2014) reported high flush rates for golden plover around the Humber estuary during the winter. Furness (1973) noted that roosting golden plovers and bar-tailed godwits at Musselburgh lagoons were much more likely to be disturbed by people than were other waders.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 200-500m
Nonbreeding season buffer zone = 200-500m
Golden plover is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for golden plover when approached by a pedestrian is median of 227m (maximum AD is 491m) during the breeding season and a maximum of 450m during the nonbreeding season. MAD values up to 200m have been suggested to protect golden plover from pedestrian disturbance during the breeding season.
In the UK, golden plover has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season; for some individuals, tolerance of human disturbance may be lower during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 200-500m is suggested to protect nesting golden plover as well as foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
AD/FID studies are required during the nonbreeding season.
Dunlin, Calidris alpina
Conservation Status
UK: Red List
European: Declining
UK status
Migrant Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 8,600-10,500 breeding pairs, 350,000 individuals in winter (Woodward et al., 2020); Scottish population = 8,000-10,000 breeding pairs (schinzii subspecies), 37,000-58,000 individuals in winter (alpina subspecies) (Forrester et al., 2012).
UK long-term trend
Decline. Breeding population declined in the Outer Hebrides by 65% between 1983-2007, there were also losses in marginal upland areas, particularly in western Ireland, northern England and southern Scotland between 1968-72 to 2007-11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Dunlin was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking over moorland in Norway: Range of mean FID = 13.1 to 81.3m (n = 20) (Byrkjedal, 1987).
Pedestrian walking/running on moorland in England: Mean AD = 30m (n = 30); Min/Max AD = 8 to 83m (Yalden and Yalden, 1989).
Surveyor walking in Scotland: Min/Max FID = 50-100m (Andy Douse, pers. obs.).
Nonbreeding season:
Surveyor walking along a shoreline in England: Mean FID = 43.9m (n = 117); Min/Max FID = 9 to 194m (Collop et al., 2016).
Surveyor walking over mudflats in Scotland: Mean FID = 163.9m (n = 4) (Dwyer, 2010).
Surveyor walking in a coastal lagoon habitat in Italy: Mean FID = 39m (n = 40); Min/Max FID = 5 to 81m (Scarton, 2018b).
Surveyor walking along mudflats in Denmark: Mean FID = 70m (n = 317), Min/Max FID = 15 to 450m (Laursen et al., 2005).
Pedestrian leisure (walking and watercraft) along the shoreline in England: Median AD = 8m (n = 11); Range of median FID = 30 to 55m (n = 23); Min/Max FID = 8 to 100m (Liley et al., 2011).
Pedestrian leisure (unspecified) along the shoreline in England: Min/Max AD = 50 to 100m; Median FID = 75m (n = 19); Min/Max FID = 25 to 300m (Liley et al., 2010).
Pedestrian walking/running on tidal flats in the Netherlands /Germany: Range of mean FID = 71 to 163m; Min/Max FID = 57 to 300m (Smit and Visser, 1993).
Motorised watercraft (motorboat) in a coastal lagoon habitat in Italy: Mean FID = 52.3m (n = 23); Min/Max FID = 9 to 175m (Scarton, 2018b).
MAD and/or
Buffer zone
Quantitative distances
Breeding:
Pedestrian walking/running in moorland habitat in England: Mean MAD = 50 to 200m (Pearce-Higgins et al., 2007).
Nonbreeding season:
Surveyor walking along a shoreline in North America: Buffer zone = 89m (Koch and Paton, 2014).
Pedestrian walking/running along footpaths or the presence of railways close to intertidal areas in England: Buffer zone = 25 to 75m, although a buffer zone of 200m may be needed to protect a mix of intertidal species (Burton et al., 2002a).
Surveyor walking in a coastal lagoon habitat in Italy: Buffer zone = 82m, but this buffer zone would increase to 270m to protect mixed species winter roosts (Scarton, 2018b).
Motorised watercraft (motorboat) in a coastal lagoon habitat in Italy: Buffer zone = 124m, but this buffer zone would increase to 270m to protect mixed species winter roosts (Scarton, 2018b).
Ecology and non-quantitative disturbance responses
One of three subspecies of dunlin breeds in the UK (schinzii ssp.) in the upland areas of Scotland, Wales and northern England (Pennines) (Balmer et al., 2013). During the breeding season, schinzii ssp. are found on wet upland and montane heath, especially where pool systems occur, but also on the machairs of the Outer Hebrides and rarely on coastal saltmarsh (Snow and Perrins, 1998). In Scotland the highest breeding densities occur on the Northern Isles, Outer Hebrides and the Flow Country of Caithness and Sutherland; in England, high breeding densities occur in the Pennines (Balmer et al., 2013). Dunlins breed on the ground concealed in vegetation, the nest is a shallow scrape lined with grass and leaves (Snow and Perrins, 1998).
Wintering dunlins are widely distributed throughout the coastlines of Britain and Ireland, the largest concentrations are on estuaries (Balmer et al., 2013). The alpina ssp. which breeds in Fennoscandia and northwest Russia, winters in western Europe, including the UK; both schinzii and arctica subspecies winter mainly in northwest Africa (Wernham et al., 2002; Snow and Perrins, 1998). Dunlins mainly spend the winter on the coast, but they can also frequent a wide variety of coastal and inland waterbodies including lagoons, muddy freshwater shores, tidal rivers, flooded fields, sewage farms, saltworks, sandy coasts, lakes and dams (BirdLife International, 2021b). Dunlins feed mainly on invertebrates; insects may chiefly be eaten during the breeding season and marine invertebrates during the nonbreeding season (Snow and Perrins, 1998). Similar to other waders, dunlins roost during high tides and at night, but this species prefers large fields of naturally fertilised short pasture or soil-based crops with few vertical structures that could be used by predators (Shepherd and Lank, 2004).
Dunlins are potentially sensitive to human disturbance during the breeding season. As a ground nesting species, dunlin is vulnerable to predator disturbance; Jackson (2001) showed that hatching success can be increased by excluding ground predators with fences around nesting areas. This species can be disturbed by human recreational activity taking place over their breeding grounds, although in the Peak District, Pearce-Higgins et al. (2007) found that, like golden plover, the provision of well-surfaced paths in breeding areas that receive at least 30 visitors a day can reduce the impact of human disturbance on the breeding success of this species. Yalden and Yalden (1989) suggest that dunlins are less sensitive to human intruders on their territories compared with golden plovers. Dunlins are relatively small birds and, like many other wader species, have cryptic plumage colour (Ferns, 2003) that can make them difficult to see on the ground, especially in amongst vegetation. For this reason, dunlins are more often detected in flight or when calling and estimating AD for this species is difficult.
During the nonbreeding season, reports of disturbance on dunlins are mixed. Kirby et al. (1993) found dunlin to be one of the more commonly disturbed species at roost sites on the Welsh Dee Estuary and tended to leave it altogether when disturbed by dogs and walkers. Davidson and Rothwell (1993) did not include it among the more nervous species (compared with redshank, bar-tailed godwit and curlew), and Burton et al. (2002a) recorded that it was the last species to fly when disturbed by walkers, although counts were still significantly lower at sites close to footpaths (see literature review in Woodward et al., 2015). Burton et al. (2002b) also noted that dunlin is threatened by disturbance on intertidal mudflats from construction work in the UK. Furness (1973) noted that roosting dunlins at Musselburgh lagoons were much less likely to be disturbed by people or aircraft than were bar-tailed godwits or golden plovers.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 150-300m
Dunlin is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for dunlin when approached by a pedestrian is 100m (maximum AD is 83m) during the breeding season and 450m during the nonbreeding season. For motorised watercraft, a range of mean FID values between 9-175m have been recorded during the nonbreeding season.
MAD values up to 200m have been suggested to protect dunlin from pedestrian disturbance during the breeding season. During the non-breeding season, buffer zones ranging between 25 to 89m have been proposed to protect dunlin against pedestrian disturbance, but for mixed winter flocks, it has been suggested that buffer zones should be larger between 200 to 270m. To protect against motorised watercraft disturbance, a 124m buffer has been proposed to protect dunlin during the nonbreeding season, but for flocks of mixed waders containing more sensitive species (e.g. curlew), a buffer zone of 270m is suggested to protect winter roosts.
In the UK, dunlin has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season; tolerance of human disturbance may be lower during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 100-200m is suggested to protect nesting dunlin and a buffer zone of 150-450m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
Current studies provide a good range of FID values. Future studies should specify habituation to disturbance when recording AD/FID.
Red knot, Calidris canutus
Conservation Status
UK: Amber List
European: Least Concern
UK status
Passage/Winter Visitor
UK and Scottish population estimate
UK winter population = 265,000 individuals (Woodward et al., 2020); Scottish winter population = 20,400-25,800 individuals (Forrester et al., 2012).
UK long-term trend
Slight increase. Wintering range increased by 27% in Britain and 58% in Ireland between 1981/84 to 2007/11, the population has increased by 15% between 1983/84 and 2008/09 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Red knot was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: FID = 26m (n = 1) (Díaz et al., 2021).
Surveyor walking in Europe: FID 26m (n = 1) (Jiang and Møller, 2017).
Nonbreeding season:
Surveyor walking over mudflats in Scotland: FID = 60.01m (n = 1) (Dwyer, 2010).
Surveyor walking along a shoreline in England: Mean FID = 71.8m (n = 78); Min/Max FID = 20 to 240m (Collop et al., 2016).
Surveyor walking in a range of habitats in Australia: Mean FID = 21.3m (n = 8) (Weston et al., 2012).
Pedestrian (general) along a shoreline in Australia: Mean FID = 74.4m (Lilleyman et al., 2016).
Pedestrian leisure (unspecified) along the shoreline in England: FID = 51m (n = 1) (Liley et al., 2010).
Non-motorised watercraft (rowing boat) in nearshore waters off Denmark: Mean FID = 260m (Laursen et al., 2017).
Motorised watercraft (motorboat) in nearshore waters off Denmark: Mean FID = 200m (Laursen et al., 2017).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season:
Pedestrian (general) along a shoreline in Australia: Buffer zone = 100m (Lilleyman et al., 2016).
Pedestrian walking/running along footpaths close to intertidal areas in England: Buffer zone = 150m, although a buffer zone of 200m may be needed to protect a mix of intertidal species (Burton et al., 2002a).
Ecology and non-quantitative disturbance responses
Red knot are winter visitors and passage migrants to the UK; this species breeds in the high Arctic in Greenland and Canada (Balmer et al., 2013). During the nonbreeding season, birds migrate to northwest Europe; over 65% of the population overwinters in the UK where it is strictly a coastal species (Balmer et al., 2013). The distribution of knot is widespread around most of the UK, the highest concentrations are found on muddy and sandy shores, especially in estuaries (the Wash is an internationally important site), but this species is generally absent from northern and western Scotland (Balmer et al., 2013). In Scotland birds can be found throughout the year due to birds on passage and failed breeders returning to wintering grounds early (Snow and Perrins, 1998). Outside the breeding season, red knot feed mainly on intertidal invertebrates, chiefly molluscs (Snow and Perrins, 1998).
Among shore birds, red knot has long been known to be highly vulnerable to human disturbance, particularly at their roost sites (Woodward et al., 2015; Furness, 1973; Mitchell et al., 1988). Like other members of the Scolopacidae family, knot roost together at high tide on undisturbed rocks, sandy spits or offshore islets (Snow and Perrins, 1998). Furness (1973) found that red knot on the Forth Estuary will fly to another roost approximately 10 miles away if disturbance is high enough. Mitchell et al., (1988) showed that numbers of knot fell by 79% at roosts on the Welsh Dee Estuary between 1979/80 to 1985/86 and that birds moved to disturbance-free sites on the Alt Estuary; for some knots disturbance (particularly from dogs, horse-riders and walkers) at their roost could result in an extra round trip of approximately 25 miles which may account for 14% of their daily energy expenditure. Kirby et al. (1993) also note that knot tended to leave the Dee Estuary altogether when disturbed by dogs and walkers. Burton et al. (1996) suggested that after redevelopment at Hartlepool West Harbour, Cleveland, the numbers of wintering knot declined despite the creation of a new island roost, likely because of increased disturbance, particularly from people and boats due to the increased access to the marina. Pfister et al. (1992) suggested that the severity of the impact of human disturbance on knot at Plymouth Beach is probably most evident in their long-term decline in abundance at that site.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Medium evidence
Nonbreeding season buffer zone = 100-300m
Red knot is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for knot is 240m when approached by a pedestrian and a mean of 260m when approached by a non-motorised watercraft during the nonbreeding season; the majority of mean FID values are under 100m when approached by a pedestrian. The maximum FID value recorded for knot when approached by a pedestrian during the breeding season is 26m, but as this species does not breed in the UK, quantitative values recorded during the breeding season may not be relevant to disturbance in the UK. A buffer zone up to 150m has been suggested to protect knot from pedestrian disturbance during the nonbreeding season, but in flocks of mixed waders during the nonbreeding season containing more sensitive species, a larger buffer zone up to at least 200m may be required to protect against disturbance.
In the UK, red knot has the potential to be disturbed on foraging and roosting grounds during the nonbreeding season; tolerance of human disturbance may be lower at roost sites. A buffer zone of 100-300m is suggested to protect nonbreeding knot from pedestrian disturbance.
Knowledge gaps
Lack of studies specifying AD/FID at roost sites during the nonbreeding season.
Purple sandpiper, Calidris maritima
Conservation Status
UK: Red List, Schedule 1
European: Least Concern
UK status
Scarce Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 1 breeding pair in Scotland, 9,900 individuals in winter (Woodward et al., 2020); Scottish winter population = 16,000 individuals (Forrester et al., 2012). Scottish breeding population may have decreased since Forrester et al. (2012) estimated 1-5 pairs.
UK long-term trend
Eaton et al. (2021) state a strong decrease in breeding birds (-67%) over 25 years.
Determining trends for this species is difficult due to difficulties with data comparison (Balmer et al., 2013). However, the UK wintering population recorded at the open-coast decreased by 27% between 1984/85 - 2006/07; Irish population declined by 33% between 1987/88 - 1997/98 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Purple sandpiper was not included in Ruddock and Whitfield (2007).
No AD/FID distances available for purple sandpiper.
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone available for purple sandpiper.
Ecology and non-quantitative information on disturbance responses
Purple sandpiper is a very rare breeding species in the UK, confined to two breeding sites in the Cairngorms National Park, Scotland, where it breeds at the southernmost edge of the species’ Arctic range (Balmer et al., 2013; Forrester et al., 2012). In these locations, very small numbers of purple sandpiper breed on mountains above 1,000m; adults and young occupy habitat beside the wet margins of streams, flushes and pools (Forrester et al., 2012). Like many other waders, purple sandpiper is an open ground nesting species, the nest is a small cup part filled with leaves (Snow and Perrins, 1998).
Purple sandpiper is primarily a winter visitor to the UK, it is found on all coasts where there is suitable habitat, but it prefers exposed, shallow rocky coastlines (Balmer et al., 2013; Wernham et al., 2002). In the UK, this species is the most northerly of wintering waders, density is highest along the coasts of the northern North Sea, Northern Isles and Outer Hebrides as well as exposed headlands in Ireland; southern England and Wales hold small populations and relatively few birds use estuaries (Balmer et al., 2013). Three separate breeding populations winter around the coasts of the UK, the majority of the northern and western birds breed in Canada whilst those wintering in eastern Britain originate from breeding populations in Scandinavia and Svalbard (Balmer et al., 2013). Purple sandpipers feed both during the day and at night in the littoral zone, the winter diet of this species is largely composed of small winkles and blue mussels, kelp flies are also hunted for amongst seaweed (Forrester et al., 2012).
Dierschke (1994) found that purple sandpipers spend only about half as long foraging during winter as do other wader species, it has been noted that this species will not forage during rising tides, also high tides during daylight hours restricts the foraging period (Simon Cohen, pers. comm.). Burton and Evans (1997) concluded that the predictable food supply on rocky shores allows purple sandpipers to achieve higher survival rates than estuarine waders. These features suggest that purple sandpipers are likely to be much less vulnerable to adverse effects from human disturbance. In addition, purple sandpipers are less prone to being disturbed by human presence than are most wader species, possibly because of their crypsis and the greater opportunity to remain undetected in rocky shore habitat compared with waders that frequent open mud or sand. Indeed, purple sandpipers tend to crouch on the rocks as a pedestrian approaches, only flying off if the person comes very close (perhaps within about 5 to 8 m). Cramp and Simmons (1982) describe purple sandpiper as “noted for tameness throughout the year”. Baxter and Rintoul (1953) state “the purple sandpiper is one of the tamest of the waders, it will sit drowsily by the side of the sea until one is within a few feet of it”.
Although this review has been unable to find FID data for purple sandpiper, the literature indicates that this will be smaller than for most estuarine waders.
Likely sensitivity to disturbance = Low/Medium
Quantitative information = No evidence
Breeding season buffer zone <300m
Nonbreeding season buffer zone <300m
Purple sandpiper is assessed to have a low to medium sensitivity to human disturbance.
There are a lack of disturbance studies and recommended buffer zones for purple sandpiper. Due to the scarcity and remote locations of breeding purple sandpipers in the UK, this species is unlikely to be encountered on breeding grounds by humans. Non-quantitative studies suggest that buffer zones required to protect purple sandpiper during the nonbreeding season may be lower than those for estuarine waders.
In the UK, purple sandpiper mainly has the potential to be disturbed on foraging and roosting grounds during the nonbreeding season. From studies on other wader species, a buffer zone <300m is suggested to protect breeding and nonbreeding purple sandpiper from pedestrian disturbance.
Knowledge gaps
Lack of studies providing AD/FID values during the nonbreeding season.
Wood sandpiper, Tringa glareola
Conservation Status
UK: Amber List, Schedule 1
European: Least Concern, Annex 1
UK status
Scarce Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 30 breeding pairs in Scotland (Woodward et al., 2020); Scottish passage population = 10-50 individuals during spring and 20-50 individuals during autumn (Forrester et al., 2012). Scottish population estimate has increased since Forrester et al. (2012) estimated a breeding population of 18-21 pairs.
UK long-term trend
Eaton et al. (2021) state a strong increase in breeding birds (+528%) over 25 years.
The small breeding population in northern Scotland has increased in range and size since 1988/91 when the population was six pairs (Balmer et al., 2013). A total of 27 breeding pairs were recorded in 2010 (Balmer et al., 2013), this has increased to 30 pairs in 2013-17 (Woodward et al., 2020).
AD/FID
Quantitative disturbance distances
FID updates (Díaz et al., 2021; Gnanapragasam et al., 2021; Mosvi et al., 2019; Jiang and Møller, 2017; Whitfield and Rae, 2014) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: Mean FID = 20.3m (n = 3), Min/Max FID = 16 to 23m (Díaz et al., 2021).
Surveyor walking in Europe: FID = 20.3m (n = 3) (Jiang and Møller, 2017).
Surveyor walking in Norway:
Mean FID of “guard” parents = 59m (n = 27), Min/Max FID = 15 to 100m;
Mean FID of “non-guard” parents = 38m (n = 14), Min/Max FID = 21 to 60m
(Whitfield and Rae, 2014)
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD = 225m (n = 5); Min/Max AD (80% opinion range) = <10 to 300m; Min/Max AD (90% opinion range) = 150 to 300m.
Range of median FID = 5 to 125m (n = 8); Min/Max FID (80% opinion range) = <10 to 300m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season:
Surveyor walking in a range of habitats in Sir Lanka: Mean FID = 33m (n = 15); Min/Max FID = 10 to 57m (Gnanapragasam et al., 2021).
Unknown season:
Surveyor walking around a lake in Pakistan: Mean FID = 33m (Mosvi et al., 2019).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (Whitfield and Rae, 2014) published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Safe working distance = 200 to 600m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Pedestrian (general activity) in Norway: Buffer zone = 160m (Whitfield and Rae, 2014).
Ecology and non-quantitative information on disturbance responses
In the UK, wood sandpiper is a rare breeding species confined to boggy habitats in Scotland; highest densities are recorded in Sutherland and Caithness, but other breeding sites have been recorded in Inverness-shire, Wester Ross and the Outer Hebrides (Balmer et al., 2013). Wood sandpipers breed mainly in marshes and swamps, usually close to lochs (Forrester et al., 2012). This species nests on the ground in amongst dense vegetation or in old tree nests of other birds (Svensson et al., 2009; Snow and Perrins, 1998). Both male and female wood sandpiper parents typically care for young chicks with a division in roles between a “guard” bird which maintains an alert posture at a “look-out” location with a clear view of the surrounding area, and a “non-guard” bird which broods and stays close to chicks (Whitfield and Rae, 2014). The diet of wood sandpiper is most likely composed of terrestrial and freshwater insects, although little is known about the diet of this species in Scotland (Forrester et al., 2012).
Wood sandpipers do not generally overwinter in the UK, after the breeding season this species migrates south to winter in Africa (Wernham et al., 2002). Wood sandpipers are recorded in Britain during passage (Balmer et al., 2013), many migrants are likely to be from the Scandinavian breeding population (Wernham et al., 2002). In Scotland, wood sandpipers recorded outside the breeding season are mostly located at inland sites beside freshwater burns and lochs; more rarely they may be recorded along the coast (Forrester et al., 2012).
Wood sandpipers are potentially susceptible to human disturbance (Kalejta-Summers and Chisholm, 2009) and this species has been described as a “wary and nervous bird” (e.g. Oiseaux-Birds, 2021; Australian Government, 2021) particularly in flocks, although solitary birds will sometimes tolerate close approach (Australian Government, 2021). Beaman and Madge, (1998) state that wood sandpipers are considered to flush easily. During the breeding season the distance at which parents with young chicks react to an approaching pedestrian depends on whether or not the birds are on guard duty. In a study in Norway, Whitfield and Rae (2014) observed that birds on guard duty reacted sooner to a surveyor approaching the nest (alarm called at a mean distance of 72m, Mean FID = 59m) than a parent not on guard duty on the nest (alarm called at a mean distance of 44m, Mean FID = 38m). Whitfield and Rae (2014) also noted that the wood sandpipers in their study area (which was not subject to any human disturbance, other than research activities) did not react to human presence between 150–200m.
Likely sensitivity to disturbance = Medium
Quantitative information = High agreement & Limited evidence
Breeding season buffer zone = 150-300m
Wood sandpiper is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for wood sandpiper is 100m when approached by a pedestrian during the breeding season. Ruddock and Whitfield (2007) suggested that the upper pedestrian disturbance limit for wood sandpiper during the breeding season is 150 to 300m. Buffer zones for wood sandpipers range from 200 to 600m for forestry operations and 160m for pedestrian disturbance during the breeding season. The maximum FID value recorded for wood sandpiper when approached by a pedestrian during the nonbreeding season is 57m, but as this species does not generally overwinter in the UK, quantitative values recorded during the nonbreeding season may not be relevant to disturbance in the UK.
In the UK, wood sandpiper has the potential to be disturbed on breeding grounds.
A precautionary buffer zone of 150-300m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect nesting wood sandpiper from pedestrian disturbance.
Knowledge gaps
Further AD/FID studies required during the breeding season investigating a range of disturbance sources.
Common redshank, Tringa totanus
Conservation Status
UK: Amber List
European: Declining
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 22,000 breeding pairs, 100,000 individuals in winter (Woodward et al., 2020); Scottish population = 11,700-17,500 breeding pairs, 4,000-25,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
Strong Decline. There has been a 44% contraction of breeding range across the UK between 1968-72 to 2007-11, losses in range and abundance reflect a 39% population decline in the UK between 1995-2010 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Redshank was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Scotland: FID = 21 (n = 1) (Díaz et al., 2021).
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 19 to 41.3m (n = 16); Min/Max FID = 12 to 57m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Spain: FID = 18m (n = 1) (Díaz et al., 2021).
Surveyor walking in a coastal lagoon habitat in Italy: Mean FID = 39m (n = 20); Min/Max FID = 21 to 55m (Scarton, 2018a).
Surveyor walking in Europe: Mean FID 27.8m (n = 19) (Jiang and Møller, 2017).
Nonbreeding season:
Surveyor walking over mudflats in Scotland: Mean FID = 149.9m (n = 43) (Dwyer, 2010).
Surveyor walking along a shoreline in England: Mean FID = 79.8m (n = 53); Min/Max FID = 28 to 187m (Collop et al., 2016).
Surveyor walking along mudflats in Denmark: Mean FID = 137m (n = 73), Min/Max FID = 40 to 450m (Laursen et al., 2005).
Surveyor walking around inland waterbodies in Africa: Range of mean FID = 24 to 38.7m (n = 5), Min/Max FID = 22 to 41m (Mikula et al., 2018).
Surveyor walking in Europe: Mean FID = 4.74m (n = 2) (Møller and Erritzøe, 2010).
Surveyor walking in Europe: Mean FID = 29.71m (n = 7) (Møller, 2008a).
Pedestrian leisure (bait digging) along tidal flats in England: FID = 22m (n = 1) (Fearnley et al., 2013).
Pedestrian leisure (walking and watercraft) along the shoreline in England: Median AD = 60m (n = 15); Range of median FID = 30 to 70m (n = 51); Min/Max FID = 10 to 130m (Liley et al., 2011).
Pedestrian leisure (unspecified) along the shoreline in England: Min/Max AD = 20 to 125m; Median FID = 44.5m (n = 78); Min/Max FID = 10 to 150m (Liley et al., 2010).
Pedestrian walking/running along a shoreline in Ireland: Mean FID = 37m (n = 29) (Fitzpatrick and Bouchez, 1998).
Surveyor walking in a range of habitats in Sir Lanka: Mean FID = 33 (n = 26); Min/Max FID = 15 to 55m (Gnanapragasam et al., 2021).
Non-motorised watercraft (kayak) in nearshore waters off Denmark: Mean FID = 175m (Laursen et al., 2017).
Non-motorised watercraft (wind surfer) in nearshore waters off Denmark: Mean FID = 260m (Laursen et al., 2017).
Unknown season:
Surveyor walking around a lake in Pakistan: Mean FID = 37m (Mosvi et al., 2019).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Surveyor walking in a coastal lagoon habitat in Italy: Buffer zone = 55m. Conservative buffer zone of 100m is proposed (Scarton, 2018a).
Pedestrian walking/running along footpaths close to intertidal areas in England: Buffer zone = 50m, although a buffer zone of 200m may be needed to protect a mix of intertidal species (Burton et al., 2002a).
Ecology and non-quantitative disturbance responses
Redshank has a patchy breeding distribution in Scotland, England and Northern Ireland. The species breeds in a variety of damp habitats including coastal marshes, lowland wet grasslands and rough pasture on moorland fringes (Balmer et al., 2013). In Scotland the highest breeding densities occur on the Northern Isles, Outer Hebrides and in Caithness; in England, high breeding densities occur in the Pennines, Lancashire and on the coastal marshes of southeast England (Balmer et al., 2013). Redshank is a ground nesting species, the nest is a shallow scrape in amongst short vegetation and/or tussocks and is lined with vegetation (Snow and Perrins, 1998).
Wintering redshanks are widely distributed throughout the coastlines of Britain and Ireland, the largest concentrations are on estuaries and the Northern Isles (Balmer et al., 2013). Redshanks can feed on a wide range of prey species, but the majority of the diet is made up of crustaceans, molluscs and polychaete worms on estuaries and earthworms and cranefly larvae inland (Snow and Perrins, 1998).
In common with other waders, redshank may be frequently disturbed by human activities on more urbanised wintering sites. The flight distance when disturbed by humans may be lower for redshank compared with some other wader species, especially if redshank are habituated to activities that might cause disturbance (Fitzpatrick and Bouchez, 1998). However, redshanks are considered to be susceptible to disturbance from construction and other activities and this species often feeds closer to shore than other waders (see literature review in Woodward et al., 2015). Disturbance from construction work around Cardiff Bay was found to significantly reduce the densities and feeding activity of redshank on adjacent intertidal mudflats (Burton et al., 2002b). Work by West et al. (2002) and Goss-Custard et al. (2006) has aimed to quantify the impacts of disturbance on the wader mortality rates. In the UK, populations of redshank breeding on saltmarshes declined by >50% between 1985 and 2011 which has been linked to nest trampling disturbance by grazing cattle (Sharps et al., 2017).
Redshanks, as with all waders, usually roost on the coast at high tide (BirdLife International, 2021b), but this species is also known to roost communally at inland sites including disturbed sites at a sport centre and an oil terminal complex (CAWOS, 2019). Response to disturbance at roost sites varies between individuals, Davidson and Rothwell (1993) report that redshanks roosting in narrow tidal creeks with frequent passers-by on the shore may tolerate people within 20m, yet this species on some large estuaries will take flight when a person is still over 100m away (Smit and Visser, 1993). Davidson and Rothwell (1993) considered that redshanks are one of the more nervous species of wader (in addition to bar-tailed godwit and curlew), compared with oystercatcher, turnstone and dunlin.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Robust evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 200-300m
Common redshank is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for redshank when approached by a pedestrian is 57m during the breeding season and 450m during the nonbreeding season. When approached by non-motorised watercraft during the nonbreeding season, the maximum FID recorded for redshank is a mean of 260m. In the UK, a buffer zone of 50m has been proposed to protect redshank against pedestrian disturbance during the nonbreeding season, but this buffer zone may need to be increased to 200m to protect a mix of intertidal species. A buffer zone of 100m has been suggested to protect redshank from pedestrian disturbance during the breeding season in Italy.
In the UK, redshank has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season; tolerance of human disturbance may be lower at roost sites. Depending on the level of habituation to disturbance, a buffer zone of 100-200m is suggested to protect nesting redshank and a buffer zone of 200-300m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
Further studies recording AD/FID from a range of disturbance sources during the breeding season are required.
Greenshank, Tringa nebularia
Conservation Status
UK: Amber List; Schedule 1
European: Least Concern
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 1,100 breeding pairs in Scotland, 920 individuals in winter (Woodward et al., 2020); Scottish winter population = 50-90 individuals in winter (Forrester et al., 2012).
UK long-term trend
Increasing. According to Balmer et al. (2013), greenshank breeding range has expanded by 2% since 1968-72 and 2007-11; the range of nonbreeding birds has expanded by 48% in Britain and 13% in Ireland since 1981-84 and 2007-11. Gains are most evident in Scotland and eastern England and related to increased abundance, probably as a result of milder climatic conditions (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Greenshank was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 30 to 45.5m (n = 4); Min/Max FID = 20 to 53m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Norway: FID = 30m (n = 1) (Díaz et al., 2021).
Surveyor walking in a rural habitat in Finland: FID = 84m (n = 1) (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID 36.2m (n = 5) (Jiang and Møller, 2017).
Surveyor walking in Scotland: Min/Max AD = 200-500m; Min/Max FID = 100-300m (Andy Douse, pers. obs.).
Nonbreeding season:
Surveyor walking over mudflats in Scotland: FID = 494.17m (n = 1) (Dwyer, 2010).
Surveyor walking in Europe: Mean FID = 30m (n = 2) (Møller and Erritzøe, 2010).
Surveyor walking along mudflats in Denmark: Mean FID = 94m (n = 35), Min/Max FID = 38 to 250m (Laursen et al., 2005).
Surveyor walking in a wetland habitat in Denmark: Mean FID = 78m (n = 32) (Bregnballe et al., 2009).
Surveyor walking in Africa: Mean FID = 51.3m (n = 27) (Weston et al., 2021).
Surveyor walking in a shorebird habitat in Australia: Mean FID = 55.41m (n = 17); Min/Max FID = 25 to 145m (Glover et al., 2011).
Surveyor walking in a range of habitats in Australia: Mean FID = 47.60m (n = 7) (Weston et al., 2012).
Surveyor walking in a variety of habitats: Mean AD = 55.1m (n = 7) (Blumstein et al., 2004).
Surveyor walking in a variety of habitats in Australia: Mean FID = 70.0m (n = 3) (Paton et al., 2000).
Pedestrian walking/running near inland waterbodies in Australia: Mean AD = 95m; Mean FIS = 75m (Taylor, 2006).
Surveyor walking in a range of habitats in Sir Lanka: Mean FID = 29.4 (n = 8); Min/Max FID = 21 to 36m (Gnanapragasam et al., 2021).
Pedestrian leisure (unspecified) along the shoreline in England: FID = 40m (n = 2) (Liley et al., 2010).
Animal (dogs) disturbance in Australia: Mean FID = 80.3m (n = 2) (Paton et al., 2000).
Watercraft (surveyor in an unspecified boat) in Australia: Mean FID = 60.7m (n = 3) (Paton et al., 2000).
Non-motorised watercraft (surveyor canoeing) in Australia: Mean FID = 51.5m (n = 2) (Paton et al., 2000).
Drone (surveyor operating a drone) in France: Min/Max AD = 4 to 10m (n = 5); Min/Max FID = 4 to 10m (n = 2) (Vas et al., 2015).
Unknown season:
Surveyor walking around a lake in Pakistan: Mean FID = 35m (Mosvi et al., 2019).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season:
Surveyor walking along a shoreline in Africa: Mean MAD = 40m (n = 7) (Boer and Longamane, 1996).
Pedestrian walking/running near inland waterbodies in Australia: MAD = 75 to 95m (Taylor, 2006).
Ecology and non-quantitative disturbance responses
Common greenshank is an uncommon breeding species in Scotland which is on the western edge of the world breeding range of this species (Balmer et al., 2013; Wernham et al., 2002). In Scotland, greenshanks are largely restricted to the bogs and moors of the northwest Highlands and Hebridean islands; the highest densities are in Sutherland, Wester Ross, Lewis, Harris and North Uist (Balmer et al., 2013). Greenshank is a ground nesting species; the nest is a shallow scrape made between rocks/tussocks/dead tree stumps and is located in the open, within and on the edge of native and non-native coniferous forests (Forrester et al., 2012; Snow and Perrins, 1998). Breeding greenshanks are highly site-faithful and may even use the same nest scrape in consecutive years (Wernham et al., 2002). Males are highly territorial and perform song flights high into the sky over the breeding site (Forrester et al., 2012).
Common greenshank is a migratory species; birds breeding in Palaearctic regions migrate south during the nonbreeding season (Wernham et al., 2002).
Although the movements of nonbreeding Scottish birds are not well understood (Wernham et al., 2002), most greenshanks move to coastal areas near breeding regions during the nonbreeding season (Forrester et al., 2012). Passage birds are more widespread in the UK, found in all coastal regions as well as inland, but wintering birds are more concentrated to the south and west (Balmer et al. 2013; Forrester et al., 2012). The highest concentrations of wintering greenshank are found on key estuaries throughout the UK especially in Ireland and parts of western Scotland, where birds are more widely distributed; recent gains have been recorded in eastern England and Ireland (Balmer et al., 2013). Nonbreeding greenshanks feed mainly on invertebrates and small fish (Snow and Perrins, 1998).
Greenshanks are regarded as potentially vulnerable to human disturbance, particularly when disturbance coincides with areas of habitat change. This species has probably been negatively affected by the long-term, extractive human use of moorlands by grazing, burning, hunting and forestry (RSPB, 2021a). Mason et al. (2021) suggest that moorland species in Britain such as common greenshank have probably been negatively affected by the long-term, extractive human use of moorlands by grazing, burning, hunting and forestry. Reduction of suitable moorland breeding habitat has occurred in the Flow County of Caithness and Sutherland through commercial afforestation (Forrester et al., 2012). Greenshank is threatened by the degradation and loss of wetland habitats through environmental pollution, reduced river flows and human disturbance in the Yellow Sea; in Europe greenshank may be affected by habitat degradation caused by off-road vehicles or dry conditions (BirdLife International, 2021b).
Breeding greenshanks are considered to be shy and to have highly cryptic behaviour, presumably in response to predation risk (Nethersole-Thompson 1951). Similar to golden plover, the distance at which greenshank are likely to fly away from human disturbance may depend on how conspicuous the disturbance is (e.g. a walker appearing against a skyline may cause more disturbance than a walker hidden in a valley) and the predictability of the source of disturbance. Gilbert et al. (1998) recommended to keep disturbance to a minimum for survey work and suggest that there is no need to search for nests or to get close to adults; adults with young chicks are likely to be disturbed when pool systems and lochs are checked in June.
Likely sensitivity to disturbance = Medium/High
Quantitative information = High agreement & Robust evidence
Breeding season buffer zone = 300-500m
Nonbreeding season buffer zone = 300-500m
Greenshank is assessed to have a medium to high sensitivity to human disturbance.
AD and FID values recorded for greenshank are wide ranging. The maximum AD value when approached by a pedestrian is 500m during the breeding season. The maximum FID value when approached by a pedestrian is 300m during the breeding season and 494m during the nonbreeding season. The majority of recorded FID values are lower than these maximum values which likely relate to differences in habitat. During the nonbreeding season, mean FID values between 51.5 to 60.7m have been recorded for watercraft disturbance and a maximum FID of 10m has been recorded for a drone.
MAD between 40 (mean value) and 95m (maximum value) have been suggested in Africa and Australia respectively for greenshank during the nonbreeding season, although no studies have yet recommended buffer zones for this species in the UK.
In the UK, greenshank has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 300-500m is suggested to protect nesting greenshanks as well as foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
More AD/FID studies are required during the breeding season. Future studies should specify habituation to disturbance when recording AD/FID
Black-tailed godwit, Limosa limosa
Conservation Status
UK: Red List, Schedule 1
European: Near Threatened
UK status
Migrant Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 53 breeding pairs (mainly limosa subspecies), 41,000 individuals in winter (Woodward et al., 2020); Scottish population = 5-11 breeding pairs (islandica subspecies), 300-600 individuals in winter, 1,000+ individuals during spring and autumn passage (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a stable number of breeding birds (+9%) over 25 years.
Winter range of the islandica subspecies has expanded by 177% and 55% in Britain and Ireland respectively between 1981/84 - 2007/11, this is linked to a sustained breeding population increase in Iceland; expansion may be linked to climatic and habitat changes on breeding and wintering grounds (Balmer et al., 2013). In contrast, the subspecies limosa which breeds in England has decreased and fluctuated since the 1970s (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Black-tailed godwit was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in Europe: FID = 46.5m (n = 1) (Jiang and Møller, 2017).
Surveyor walking around a lagoon in Denmark: Mean FID = 72 to 95m (n = 203) (Holm and Laursen, 2009).
Nonbreeding season:
Surveyor walking in a range of habitats in Australia: Mean FID = 21m (n = 6) (Weston et al., 2012).
Surveyor walking in a shorebird habitat in Australia: Mean FID = 31.25m (n = 4); Min/Max FID = 27 to 35m (Glover et al., 2011).
Pedestrian (general) along the shoreline in England: AD = 125 (n = 1); Min/Max FID = 30 to 150m (n = 3) (Liley et al., 2010).
Surveyor walking in a range of habitats in Sir Lanka: Mean FID = 36.9 (n = 7); Min/Max FID = 18 to 46m (Gnanapragasam et al., 2021).
Unknown season:
Surveyor walking around a lake in Pakistan: Mean FID = 36m (Mosvi et al., 2019).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season:
Pedestrian walking/running along footpaths or the presence of railways close to intertidal areas in England: Buffer zone = 50 to 75m, although a buffer zone of 200m may be needed to protect a mix of intertidal species (Burton et al., 2002a)
Ecology and non-quantitative information on disturbance responses
Small numbers of black-tailed godwit breed in the UK. In England, the nominate subspecies limosa is associated with increasingly modified agricultural areas, breeding in lowland wet grasslands and flood meadows (Forrester et al., 2012); the main breeding areas are located in East Anglia, but confirmed breeding records of this subspecies have also been recorded in Lancashire, Yorkshire and Kent (Balmer et al., 2013). Very small numbers of the islandica subspecies mainly breed in Orkney and Shetland on moorland with a preference for wet marshland and mesic grasslands (Balmer et al., 2013; Forrester et al., 2012). Black-tailed godwit is a ground nesting species, nests are a shallow scrape lined with stems and leaves located in short vegetation (Snow and Perrins, 1998).
In the nonbreeding season, resident black-tailed godwits are joined by large numbers of the Iceland breeding islandica subspecies (Balmer et al., 2013). Overwintering birds are scattered around the UK, the highest densities are found in coastal areas around East Anglia, the Thames Basin, North Wales, northwest England, the east and south Irish coasts and the Shannon Estuary; this species is generally absent on the west coast of mainland Scotland (Balmer et al., 2013). Most of the overwintering population is composed of the islandica subspecies which has a preference for coastal estuaries (although they may also inhabit inland sites); the resident limosa subspecies prefers to winter at inland freshwater sites (Balmer et al., 2013; Forrester et al., 2012). Black-tailed godwits feed chiefly on invertebrates during the winter and migration periods, plant material may also be consumed (Snow and Perrins, 1998).
Black-tailed godwits appear to be able to habituate to some types of human presence and may have a relatively high level of tolerance towards human disturbance, particularly during the nonbreeding season. Burton et al. (2002a) considered overwintering black-tailed godwit to be one of the most tolerant species to walkers along footpaths in estuaries in England at low tide, although numbers were still significantly lower at sites close to a footpath. In a similar study on English east coast estuary sites, Gill et al. (2001) found no evidence that human presence reduced the number of black-tailed godwits; the authors also found that the presence of marinas or footpaths did not impact the number of godwits supported on the adjacent mudflats. A study investigating human disturbance on black-tailed godwit, curlew and teal in Co. Cork, Ireland, found that out of the three species, black-tailed godwits were the least affected by disturbance events and were likely to move <50m from their original position when a disturbance event occurred (Sexton, 2017). Birds at high tide roosts are considered to be susceptible to disturbance (Davidson and Rothwell 1993), but Percival (2011) found that roosting black-tailed godwits in the Humber appear to be tolerant of a relatively high disturbance environment. Percival (2011) found that black-tailed godwits roost at high tide on the North Killingholme Haven Pits which are located in an area adjacent to the Humber Sea Terminal and to car import compounds; there was no evidence found in this study that industrialisation had reduced the ability of the pits to support the godwit population.
However, black-tailed godwit may be sensitive to disturbance during the breeding season (e.g. Frikke, 1991). In a study in the Netherlands, Reijnen et al. (1996) found that >10% of the breeding black-tailed population was lost beyond 100m of a road with 5000 cars per day. In another study in Denmark on breeding black-tailed godwits, Holm and Laursen (2009) found that one person walking the same route seven times per day in March–June reduced black-tailed godwit territory density within 300–500 m. In a management plan for black-tailed godwit (2007-2009), the European Commission suggested that this species is especially sensitive to disturbance in breeding areas, and there is a need to assess the effects of increasing disturbance on breeding success in agricultural environments (European Commission 2007b).
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 100-200m
Nonbreeding season buffer zone = 100-200m
Black-tailed godwit is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for black-tailed godwit when approached by a pedestrian is a mean of 95m during the breeding season and 150m during the nonbreeding season. A buffer zone from 50 to 75m has been suggested to protect black-tailed godwit from pedestrian disturbance during the nonbreeding season, although in flocks of mixed waders during the nonbreeding season containing more sensitive species, a 200m buffer zone may be required to protect against disturbance.
In the UK, black-tailed godwit has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 100-200m is suggested to protect both breeding and nonbreeding black-tailed godwit from pedestrian disturbance.
Knowledge gaps
More AD/FID studies are required during the breeding season and wider range of studies are required for different disturbance sources.
Bar-tailed godwit, Limosa lapponica
Conservation Status
UK: Amber List
European: Secure, Annex 1
UK status
Passage/Winter Visitor
UK and Scottish population estimate
UK winter population = 53,500 individuals (Woodward et al., 2020); Scottish winter population = 10,000-14,000 individuals (Forrester et al., 2012).
UK long-term trend
Stable. The UK wintering population has remained largely stable between 1981-84 to 2007-11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Bar-tailed godwit was not included in Ruddock and Whitfield (2007).
Breeding:
Surveyor walking in Europe: Mean FID 33.3m (n = 5) (Jiang and Møller, 2017).
Nonbreeding season:
Surveyor walking over mudflats in Scotland: Mean FID = 96.91m (n = 3) (Dwyer, 2010).
Surveyor walking along a shoreline in England: Mean FID = 84.4m (n = 92); Min/Max FID = 32 to 225m (Collop et al., 2016).
Surveyor walking in an estuary in England: Mean FID = 39m (n = 23) (Brett, 2012).
Surveyor walking along mudflats in Denmark: Mean FID = 156m (n = 120), Min/Max FID = 40 to 450m (Laursen et al., 2005).
Surveyor walking in a variety of habitats in Australia: Mean FID = 22.1m (n = 196) (Blumstein, 2003).
Surveyor walking in a variety of habitats in Australia: Mean FID = 22.1m (n = 177); Min/Max FID = 2.1 to 102.2m (Blumstein et al., 2003).
Surveyor walking in a variety of habitats in Australia: Mean FID = 48.6m (n = 2) (Paton et al., 2000).
Surveyor walking in a shorebird habitat in Australia: Mean FID = 59.50m (n = 4); Min/Max FID = 45 to 69m (Glover et al., 2011).
Surveyor walking in a range of habitats in Sir Lanka: Mean FID = 34 (n = 2); Min/Max FID = 18 to 50m (Gnanapragasam et al., 2021).
Pedestrian leisure (walking and watercraft) along the shoreline in England: AD = 30m (n = 1); FID = 25m (n = 1) (Liley et al., 2011).
Pedestrian walking/running on tidal flats in the Netherlands /Germany: Range of mean FID = 107 to 219m; Min/Max FID = 88 to 225m (Smit and Visser, 1993).
Non-motorised watercraft (kayak) in nearshore waters off Denmark: Mean FID = 200m (Laursen et al., 2017).
Non-motorised watercraft (wind surfer) in nearshore waters off Denmark: Mean FID = 230m (Laursen et al., 2017).
Watercraft (surveyor in an unspecified boat) in Australia: Mean FID = 53.5m (n = 2) (Paton et al., 2000).
Non-motorised watercraft (surveyor canoeing) in Australia: Mean FID = 41.9m (n = 2) (Paton et al., 2000).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone available for bar-tailed godwit.
Ecology and non-quantitative disturbance responses
The European bar-tailed godwit population (Limosa lapponica lapponica) breeds in the Arctic in Northern Scandinavia and around the White Sea (Balmer et al., 2013; Engelmoer, 2008). This species does not breed in the UK, although in Scotland, small numbers of immature birds remain on the coastline throughout the summer. The European population winters in Western Europe, mainly in the UK and the Western part of the Wadden Sea (Versluijs, 2011). During the nonbreeding season, bar-tailed godwit is chiefly a coastal species around the UK on low-lying shores, the largest numbers occur on major estuaries (Balmer et al., 2013). This species is largely absent from much of northern and western Scotland and elsewhere where there are sections of steep cliff coastline (Balmer et al., 2013). Bar-tailed godwits feed chiefly on invertebrates, especially on insects, molluscs, crustaceans and annelid worms (Snow and Perrins, 1998).
Bar-tailed Godwits join mixed wader roosts at high tide where they can be disturbed by human activity. This species has been described as relatively sensitive to disturbance compared to other wader species (see literature review in Woodward et al., 2015). On a high tide roost in a cultivated grassland area near the Dutch Wadden Sea, Smit and Visser (1993) showed that bar-tailed godwits were disturbed 64% of the time by human activity whereas 18% had a natural cause. Davidson and Rothwell (1993) considered that in addition to curlew and redshank, bar-tailed godwits are a more nervous wader species compared with oystercatcher, turnstone and dunlin. Kirby et al. (1993) found that like other sensitive wader species including grey plover, knot and dunlin, bar-tailed godwit tended to leave the Welsh Dee Estuary when disturbed by dogs and walkers. Collop (2016) showed that in comparison to other wader species present at Poole Harbour, bar-tailed godwit had the greatest vulnerability to the impacts of disturbance, although it was also stated that over-winter survival for this species at this site was predicted to be below 100% and the same author suggested that bar-tailed godwits on the Wash may be able to cope with a 10% reduction in time spent feeding caused by daily disturbance events. Furness (1973) noted that roosting bar-tailed godwits at Musselburgh lagoons were much more likely to be disturbed by people and aircraft than were other waders.
However, in a study on inland coastal meadows around the Dutch Wadden Sea, Versluijs (2011) suggested that wintering bar-tailed godwits may tolerate some human activity. The authors of the study found that human activity caused 29% of total disturbance whereas birds flew up earlier more often (37%) to natural causes (e.g. predators). Of the birds that reacted to human disturbance, most of the flocks were present near roads and bicycle paths; often when a tractor or truck passed by the birds they flew up and they were also regularly disturbed by stopping cars and cyclists (Versluijs, 2011).
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Medium evidence
Nonbreeding season buffer zone = 200-300m
Bar-tailed godwit is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for bar-tailed godwit is 450m when approached by a pedestrian; the majority of FID values are less than a mean of 200m when approached by a pedestrian. For non-motorised watercraft, a range of mean FID values between 42 to 230m have been recorded during the nonbreeding season. The maximum FID value recorded for bar-tailed godwit when approached by a pedestrian during the breeding season is a mean of 33.3m, but as this species does not breed in the UK, quantitative values recorded during the breeding season may not be relevant to disturbance in the UK.
In the UK, bar-tailed godwit has the potential to be disturbed on foraging and roosting grounds during the nonbreeding season. There are no published buffer zones for bar-tailed godwit, but from studies on other waders, a minimum buffer zone of 200-300m is suggested to protect foraging and roosting bar-tailed godwit during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
Current studies provide a good range of FID values. Future studies should specify habituation to disturbance when recording AD/FID.
Eurasian curlew, Numenius arquata
Conservation Status
UK: Red List
European: Vulnerable
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 58,500 breeding pairs, 125,000 individuals in winter (Woodward et al., 2020); Scottish population = 58,800 breeding pairs, 85,700 individuals in winter (Forrester et al., 2012).
UK long-term trend
Strong Decline. Breeding range contracted by 78% in Ireland and 17% in Britain over the last 40 years, there has been a 44% population decline in the UK between 1995 – 2010 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Curlew was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 40 to 65m (n = 12) (Díaz et al., 2021).
Surveyor walking in a rural habitat in Finland: Range of mean FID = 34.5 to 44.6m (n = 16) (Díaz et al., 2021).
Surveyor walking in Europe: Mean FID 57.6m (n = 10) (Jiang and Møller, 2017).
Nonbreeding season:
Surveyor walking over mudflats in Scotland: Mean FID = 235.16m (n = 36) (Dwyer, 2010).
Surveyor walking along a shoreline in England: Mean FID = 340.3m (n = 39); Min/Max FID = 88 to 570m (Collop et al., 2016).
Surveyor walking in an estuary in England: Mean FID = 88m (n = 24) (Brett, 2012).
Surveyor walking in a coastal lagoon habitat in Italy: Mean FID = 140.5m (n = 11); Min/Max FID = 59 to 305m (Scarton, 2018b).
Surveyor walking along mudflats in Denmark: Mean FID = 298m (n = 110), Min/Max FID = 58 to 650m (Laursen et al., 2005).
Surveyor walking around inland waterbodies in Africa: Range of mean FID = 50m (n = 2), Min/Max FID = 46 to 54m (Mikula et al., 2018).
Surveyor walking in Europe: Mean FID = 62.75m (n = 4) (Møller and Erritzøe, 2010).
Surveyor walking in a range of habitats in Sir Lanka: Mean FID = 44.3 (n = 8); Min/Max FID = 21 to 113m (Gnanapragasam et al., 2021).
Pedestrian leisure (bait digging) along tidal flats in England: AD = 45m (n = 1) (Fearnley et al., 2013).
Pedestrian leisure (walking and watercraft) along the shoreline in England: Range of median FID = 22.5 to 50m (n = 22); Min/Max FID = 15 to 100m (Liley et al., 2011).
Pedestrian leisure (unspecified) along the shoreline in England: Min/Max AD = 25 to 200m; Median FID = 75m (n = 37); Min/Max FID = 30 to 150m (Liley et al., 2010).
Pedestrian walking/running along a shoreline in Ireland: Mean FID = 38m (n = 41) (Fitzpatrick and Bouchez, 1998).
Pedestrian walking/running on grasslands in the Netherlands/Germany: Mean FID = 213 (Smit and Visser, 1993).
Pedestrian walking/running on tidal flats in the Netherlands /Germany: Range of mean FID = 211 to 339m; Min/Max FID = 124 to 550m (Smit and Visser, 1993).
Pedestrian egg collector in the Netherlands/Germany: Mean FID = 140m (Smit and Visser, 1993).
Agricultural activities in the Netherlands/Germany: Mean FID = 129m (Smit and Visser, 1993).
Aircraft (helicopter) in the Netherlands/Germany: Mean FID = 200m (Smit and Visser, 1993).
Animals (dogs) in the Netherlands/Germany: Mean FID = 90m (Smit and Visser, 1993).
Motorised vehicle (cars) in the Netherlands/Germany: Mean FID = 188m (Smit and Visser, 1993).
Non-motorised watercraft (kayak) in nearshore waters off Denmark: Mean FID = 220m (Laursen et al., 2017).
Non-motorised watercraft (wind surfer) in nearshore waters off Denmark: Mean FID = 400m (Laursen et al., 2017).
Motorised watercraft (motorboat) in a coastal lagoon habitat in Italy: Mean FID = 140.3m (n = 19); Min/Max FID = 70 to 205m (Scarton, 2018b).
MAD and/or
Buffer zone
Quantitative distances
Nonbreeding season:
Surveyor walking in a coastal lagoon habitat in Italy: Buffer zone = 267m, buffer zone of 270m is recommended to protect mixed species winter roosts (Scarton, 2018b).
Pedestrian walking/running along footpaths close to intertidal areas in England: Buffer zone = 200m (Burton et al., 2002a).
Motorised watercraft (motorboat) in a coastal lagoon habitat in Italy: Buffer zone = 219m, buffer zone of 270m is recommended to protect mixed species winter roosts (Scarton, 2018b).
Pedestrian walking/running on grasslands in the Netherlands/Germany: Mean MAD = 100m (Smit and Visser, 1993).
Pedestrian walking/running on salt marsh in the Netherlands/Germany: Mean MAD = 200m (Smit and Visser, 1993).
Ecology and non-quantitative disturbance responses
With the recent loss of breeding curlews from most of Ireland and parts of western Britain over the past 40 years, the distribution of breeding curlews has become patchy with losses in western Scotland, Wales and southwest England and some gains in eastern and southeast England (Balmer et al., 2013). This species breeds in upland areas, the highest concentrations are now in northern England, especially the Pennines, eastern Scotland and the Northern Isles (Balmer et al., 2013). Curlew is a ground nesting species; the nest is a large depression lined with dried grass and feathers on tussocks or low hummocks (Snow and Perrins, 1998). Curlews are site faithful and will return to the same breeding grounds each year (Wernham et al., 2002).
Curlews are present around the UK coastline throughout the year, but coastal distribution is much more widespread outside the breeding season. During the winter, resident curlews leave their upland breeding areas and most spend the winter on or near the coast as well as adjacent farmland, the highest densities are on the major estuaries (e.g. the Wash, Morecambe Bay and the Solway), in the Northern Isles and in western Ireland (Balmer et al., 2013). Curlews are also site faithful in the winter and birds seldom move between estuaries (Wernham et al., 2002). Resident birds are joined by migrant birds from continental Europe during the nonbreeding season (Wernham et al., 2002). Curlews are omnivorous, intertidal invertebrates form the main part of the diet during the nonbreeding season (Snow and Perrins, 1998).
Changes in land-use, agricultural practices and drainage of wetland areas are considered to be the causes responsible for the decline in curlew numbers in the UK (Balmer et al., 2013). Human disturbance on breeding and wintering areas (including shooting that takes place in France) is believed to be of secondary importance (European Commission, 2007c). However, studies have shown that curlews are threatened by disturbance on intertidal mudflats (BirdLife International, 2021b), walkers (Burton et al., 2002a) and the flooding of mudflats and saltmarshes for tidal barrage construction (Burton, 2006), probably through indirect mechanisms associated to reductions of food resources or access/ displacement from wintering grounds (see literature review in Woodward et al., 2015). Curlew may also be at risk from improvements to water quality which has been found to cause reductions in benthic invertebrate densities at sites close to sewage outfalls (Burton et al., 2002b).
Curlews often roost on the coast at high tide with other waders (BirdLife International, 2021b), although large numbers of curlew will also roost on fields and marshland. A study by Scarton (2018b), identified Eurasian curlew to be the most sensitive species to human approach compared with other species of roosting waders. Davidson and Rothwell (1993) considered that curlew is one of the more nervous species of wader (in addition to bar-tailed godwit and redshank), compared with oystercatcher, turnstone and dunlin; although Collop (2016) suggested that large waders such as curlew may be able to cope with a 10% reduction in time spent feeding caused by daily disturbance events on the Wash. Furness (1973) noted that roosting curlews and bar-tailed godwits at Musselburgh lagoons were much more likely to be disturbed by aircraft than were other waders. A study investigating human disturbance on curlew, black-tailed godwit and teal in Co. Cork, Ireland, found that out of the three species, curlews were more susceptible to being greatly disturbed by human presence and activity; curlews predominantly left the study area when disturbed by anthropogenic causes (Sexton, 2017).
Likely sensitivity to disturbance = High
Quantitative information = Medium agreement & Robust evidence
Breeding season buffer zone = 200-300m
Nonbreeding season buffer zone = 200-650m
Curlew is assessed to have a high sensitivity to human disturbance.
The maximum FID value recorded for curlew when approached by a pedestrian is a mean of 65m during the breeding season and a mean of 340m (maximum FID of 650m) during the nonbreeding season. Also during the nonbreeding season, mean FID values have been recorded for curlew disturbed by aircraft (200m), motorised vehicles (188m), motorised watercraft (205m) and non-motorised watercraft (220 to 400m).
During the nonbreeding season, mean MAD values between 100 to 200m have been suggested to protect curlew from pedestrian disturbance. Buffer zones of 200 and 267 have been proposed for pedestrian disturbance and a buffer zone of 219m has been proposed for motorised watercraft disturbance; a buffer zone of 270m is suggested to protect winter roosts.
In the UK, curlew has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season; tolerance of human disturbance may be lower at roost sites during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 200-300m is suggested to protect nesting curlew and a buffer zone of 200-650m is suggested to protect foraging and roosting birds during the nonbreeding season from pedestrian disturbance.
Knowledge gaps
Current studies provide a good range of FID values during the nonbreeding season, additional studies required for the breeding season.
Whimbrel, Numenius phaeopus
Conservation Status
UK: Red List, Schedule 1
European: Least Concern
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 310 breeding pairs in Scotland only (Woodward et al., 2020). Scottish population estimate has decreased since Forrester et al. (2012) who estimated a population of 400-500 breeding pairs.
UK long-term trend
Overall breeding range contracted by 29% between 1968/72 - 2007/11, although there was a mixture of gains and losses in northern Scotland; the breeding population fell from 410-470 pairs in the 1980s to c.290 pairs in 2009 (Balmer et al., 2013). However, winter migrant records increased by 212% between 1981/84 to 2007/11, probably as a result of milder winters (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Whimbrel was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Finland: Mean FID = 56.7m (n = 3), Min/Max FID = 25 to 90m (Díaz et al., 2021).
Surveyor walking in Europe: FID = 37.7m (n = 2) (Jiang and Møller, 2017).
Nonbreeding season:
Surveyor walking in a range of habitats: Mean FID = 37.7m (n = 28) (Blumstein, 2006).
Surveyor walking in a shorebird habitat in Australia: FID = 90m (n = 1) (Glover et al., 2011).
Surveyor walking in Africa: Mean FID = 57.2m (n = 21) (Weston et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone available for whimbrel.
Ecology and non-quantitative information on disturbance responses
In the UK, whimbrel breed in Scotland and most of the confirmed records are in Shetland which covers 76% of the range; breeding has also been confirmed in Orkney and probable breeding was recorded in the Outer Hebrides and Caithness between 2007 and 11 (Balmer et al., 2013). In Scotland, this species breeds on heathlands, blanket bog and grazed acid grassland with little heather (Forrester et al., 2012). Whimbrel is a ground nesting species, the nest is a shallow depression lined with vegetation which may be on bare ground or in short vegetation (Snow and Perrins, 1998). This species forages on invertebrates and plant material, the proportion of each depends upon location and season (Forrester et al., 2012; Snow and Perrins, 1998).
Whimbrels do not overwinter in the UK, after the breeding season, this species migrates south to winter mainly along the western and southern coasts of Africa and on the islands and coasts of the western Indian Ocean (Wernham et al., 2002). Migrating whimbrels are regularly recorded around the coast of the UK (although there is a notable absence of passage birds in northeast Scotland), passing to and from breeding grounds in Greenland, Iceland, Fennoscandia and Russia to nonbreeding grounds; migrant birds are recorded in coastal areas as well as at inland sites (the latter particularly in England) (Balmer et al., 2013).
Whimbrels are regarded as potentially vulnerable to human disturbance, although it is possibly a minor factor compared to other threats faced by this species (BirdLife International, 2021b; Forrester et al., 2012; Wilke and Johnston-González, 2010). The main threats to whimbrel in Scotland are habitat degradation and climate change (Forrester et al., 2012). However, during shorebird migration and on the wintering grounds, excessive disturbance can reduce foraging and resting time, increase energy expenditure, decrease the level of use of available habitat and perhaps indirectly increase mortality (Watts et al., 2021; Wilke and Johnston-González, 2010). In a study on migrating shorebirds in America, Forgues (2010) found that off-road vehicles driving along beaches caused a significant decline in whimbrel numbers in the study area; birds maintained a distance of at least 75m from approaching vehicles. Peters and Otis (2007) found that nonbreeding whimbrel selecting a roost site in South Carolina showed a general trend towards avoidance of boat activity within 1000m.
In a study in Columbia, Johnston-Gonzalez and Abril (2019) suggested that whimbrel roost site selection was best explained by a combination of access to feeding territories and isolation from potential sources of mainland predators, but not by avoidance of human disturbance. Watts et al. (2021) did not find that human disturbance was a widespread threat to whimbrel night roosts in north America. In an anecdotal observation in Mozambique, Allport (2016) observed that a feeding flock of 40 whimbrel responded rapidly to a drone at c.20m above the ground; the authors noted that this response was consistent with the reaction of whimbrels to threats by predators rather than normal human disturbances, which generally did not cause a significant reaction in the study area.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 100-300m
Nonbreeding season buffer zone = 100-300m
Whimbrel is assessed to have a medium sensitivity to human disturbance.
The maximum FID value recorded for whimbrel when approached by a pedestrian is 90m during both the breeding and nonbreeding seasons, although quantitative studies are limited for this species.
In the UK, whimbrel has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during migration. There are no published buffer zones for whimbrel, but from studies on other waders, a minimum buffer zone of 100-300m is suggested to protect both breeding and nonbreeding whimbrel from pedestrian disturbance.
Knowledge gaps
More AD/FID studies are required during the breeding season and wider range of studies are required for different disturbance sources.
Red-necked phalarope, Phalaropus lobatus
Conservation Status
UK: Red List, Schedule 1
European: Least Concern, Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 64 territorial breeding males (Woodward et al., 2020); Scottish population = 13-48 breeding pairs, 0-15 individuals during passage (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a strong increase in breeding birds (+267%) over 25 years.
The population of breeding red-necked phalarope in the UK seriously declined in the 19th century, this was followed by a temporary recovery in the early 20th century followed by a further decline since the 1930s (Forrester et al., 2012). The current relic breeding population has fluctuated considerably in range and size; the current range is larger compared with 1988/91, but smaller than in 1968/72 (Balmer et al., 2013). The number of breeding males ranged from 15-30 between 1978 to 2005 and 19-27 in 2010 (Balmer et al., 2013). Woodward et al. (2020) records the UK population at 64 breeding males in 2013-17. Breeding records in Ireland were not confirmed between 2007-11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Red-necked phalarope was not included in Ruddock and Whitfield (2007).
No AD/FID distances available for red-necked phalarope.
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone available for red-necked phalarope.
Ecology and non-quantitative information on disturbance responses
In the UK, red-necked phalarope breeds only in Scotland where it is a rare breeding bird at the southernmost edge of the species’ circumpolar range (Balmer et al., 2013; Wernham et al., 2002). The main breeding areas are located in Shetland with other breeding sites in the Outer and Inner Hebrides and one in northeast Scotland (Balmer et al., 2013). This species breeds in areas of open water surrounded by vegetation, in Scotland they favour pools with rich nutrient content and low acidity (Forrester et al., 2012). Red-necked phalarope is a ground nesting species, the nest is a cup-shaped depression lined with leaves and stems (Snow and Perrins, 1998). This species forages whilst swimming, wading and walking, chiefly feeding on invertebrates (Snow and Perrins, 1998).
Red-necked phalarope do not overwinter in the UK, after the breeding season this species winters pelagically, favouring upwelling areas with abundant planktonic food (Wernham et al., 2002). A small number of migrant birds are recorded each spring, mostly in central and eastern England, whilst on their way to breeding grounds in the north (Balmer et al., 2013).
The red-necked phalarope is well known to be one of the most tolerant of wild birds to human presence. Adults have been recorded brooding chicks in a human’s hand, and during migration phalaropes allow close approach by people without disturbance (Cramp and Simmons 1982). Hildén and Vuolanto (1972) state: “Observation of phalaropes is very easy due to their tameness. A stationary observer can watch birds without disturbing them at a distance of only a few meters; egg laying, for instance, has been observed at close range without the use of a hide.” According to Congreve and Freme (1930) “The remarkable tameness of this species when breeding is well known; however, one male phalarope that F met with was so ridiculously tame that it actually fed its captured youngsters as he held them in his hand”. Michael (1938) described how red-necked phalaropes on migration would feed within 1 to 2 m of people at the edge of a lake.
Jørgensen et al. (2007) showed that red-necked phalaropes that nest in association with Arctic terns Sterna paradisaea often, but not always, respond to tern ‘dreads’ caused by predators or human disturbance long before the predator or human disturbance is close enough to cause the phalaropes to flee. They considered this to indicate the important role that colonies of terns can play in providing warning and defence for breeding phalaropes against threats from predators. In most cases, the behavioural response of phalaropes to tern dreads was simply to look up to identify the cause of the tern activity.
Everett (1971) suggested that the main threats to the very small breeding population of red-necked phalaropes in Scotland were drainage of pools, flooding of nest sites, damage to pool edges by cattle, and disturbance to nesting phalaropes by birdwatchers and photographers. The rarity of the red-necked phalarope, combined with its exceptional tolerance of humans, can result in breeding birds being seriously disturbed by people who spend too long too close to birds on breeding sites. Forrester et al., (2012) update that assessment to point out that conservation management can improve pools for phalaropes, but that egg collecting and deliberate human disturbance can still be significant factors. The impact of human disturbance is, paradoxically, because these birds are both rare and exceptionally tame, and a few irresponsible birdwatchers or photographers may deliberately disturb these rare birds on nesting sites.
Likely sensitivity to disturbance = Low
Quantitative information = No evidence
Breeding season buffer zone <50m
Red-necked phalarope is assessed to have a low sensitivity to human disturbance.
There are a lack of disturbance studies recording AD/FID values for red-necked phalarope. However, non-quantitative studies suggest that buffer zones required to protect red-necked phalarope during the breeding season may be much lower than those required for other waders.
In the UK, red-necked phalarope has limited potential to be disturbed on breeding grounds. From non-quantitative studies, a buffer zone <50m is suggested to protect breeding red-necked phalarope from pedestrian disturbance.
Knowledge gaps
Lack of studies providing AD/FID values during the breeding season.
Species: Terns
Little tern, Sternula albifrons
Conservation Status
UK: Amber List; Schedule 1
European: Least Concern, Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 1,450 breeding pairs (Woodward et al., 2020); Scottish population = 331 Apparently Occupied Nests (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a stable number of breeding birds (-14%) over 15 years.
Range loss perhaps indicates there has been a shift into fewer, larger colonies (Balmer et al., 2013). Approximately stable in Scotland, though apparently declined by about 25% in England, Wales and Ireland (Forrester et al., 2012).
AD/FID
Quantitative disturbance distances
Little tern was not included in Ruddock and Whitfield (2007).
Nonbreeding season (little tern):
Surveyor walking: Mean FID = 21.5m (n = 18) (Blumstein, 2006).
Breeding season (least tern, Sterna antillarum, stand in species for little tern):
Surveyor walking towards nesting site along a shoreline in Florida: Mean FID = 59m (n = 17) (Rodgers and Smith,1995).
Surveyor walking towards nesting site in the USA: FID = 64m (n = 1) (Erwin, 1989).
MAD and/or
Buffer zone
Quantitative distances
Breeding season (least tern, Sterna antillarum, stand in species for little tern):
Surveyor walking towards nesting site along a shoreline in Florida: Buffer zone = 154 to 180m (Rodgers and Smith,1995)
Surveyor walking towards nesting site in the USA: Buffer zone = 100 to 200m. A buffer zone of 200 to 300m may be required to protect colony sites early in the season before birds are established (Erwin, 1989).
Ecology and non-quantitative disturbance responses
Little tern is a summer visitor to the UK. The majority of little terns (c.75%) breed on beaches in England, the majority are located on three sections of the coast: the Humber/Lincolnshire, East Anglia and the Solent (Balmer et al., 2013). Other colonies exist in North Wales, the Isle of Man, Orkney, the southern Outer Hebrides and the Inner Hebrides (Balmer et al., 2013). This species makes a shallow scrape on the ground for a nest and forages by plunge diving for small fish and invertebrates (Snow and Perrins, 1998). After the breeding season, little terns migrate south to overwinter off the coasts of Africa and the Arabian Peninsula (Wernham et al., 2002).
Human disturbance is one of the main factors affecting breeding success and distribution of little tern colonies in England; birds avoid sites with regular human disturbance (Balmer et al., 2013; Mitchell and Hearn, 2004; Brown and Grice, 2005). Colonies subject to frequent human disturbance have often been abandoned by little terns in favour of areas away from human activity.
On the other hand, there have been examples of little terns taking to nest on flat gravel-covered roofs (where of course they avoid human disturbance despite people being active on the ground below and adjacent to the buildings). Foraging little terns often patrol along the shore a few metres from land, and in such situations can fly close to people without showing any strong response, so human disturbance of foraging little terns is less likely to be a problem than disturbance of birds at nests (Bob Furness, pers. obs.). Little terns do not attack people and nest in small numbers in scattered colonies; the apparent relatively low sensitivity of individuals to disturbance compared to high impact of human disturbance at colonies probably arises because people are often unaware that they are walking into a little tern colony; nests tend to be both cryptic and scattered, and adult behaviour tends to be cryptic when people are close to nests.
Likely sensitivity to disturbance = Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 100-300m
Little tern is assessed to have a medium sensitivity to human disturbance at breeding colonies, although away from breeding grounds, sensitivity is considered to be low.
There are no AD/FID records available for little tern during the breeding season, but the maximum FID value recorded for least tern when approached by a pedestrian during the breeding season is 64m. Buffer zones between 100 and 200m have been proposed to protect least terns from pedestrian disturbance during the breeding season, a larger buffer between 200 to 300m is suggested to protect colony sites early in the season before birds are established .
In the UK, little tern has the potential to be disturbed at breeding colonies. A minimum buffer zone of 100m is suggested to protect little tern colonies from pedestrian disturbance, but this may need to be increased to 300m to avoid disturbance early in the breeding season (i.e. during egg laying).
Knowledge gaps
Lack of studies on little tern providing AD/FID values during the breeding season.
Sandwich tern, Thalasseus sandvicensis
Conservation Status
UK: Amber List
European: Least Concern, Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 14,000 (13,000-15,000) breeding pairs, 65 individuals in winter (Woodward et al., 2020); Scottish population = 1,100 Apparently Occupied Nests, 500 to 5,000 individuals during passage periods (Forrester et al., 2012).
UK long-term trend
Wide annual fluctuation in colony size due to variation in the proportion of adults breeding, but overall, there has been a 23% contraction in range since 1968-72 (Balmer et al., 2013). Colonies have been lost, particularly in eastern Scotland,
with increasing proportions of the breeding population at just one site (Sands of Forvie NNR) (Forrester et al., 2012).
AD/FID
Quantitative disturbance distances
Sandwich tern was not included in Ruddock and Whitfield (2007).
No AD/FID records for sandwich tern.
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone records for sandwich tern.
Ecology and non-quantitative disturbance responses
Sandwich tern is a summer visitor to the UK. This species breeds in a small number of large colonies patchily distributed around the coasts of Britain and Ireland; some of the highest densities are recorded in northeast Scotland, Northumberland and Norfolk (Balmer et al., 2013). Colonies are largely absent along the coast of northwest Scotland, central and southern Wales and southwest England (Balmer et al., 2013). Sandwich terns nest on exposed open ground at the coast and on inshore islands, they generally select areas that are distant from human activity. This species makes a nest of a shallow scrape on the ground and forages by plunge diving for fish (Snow and Perrins, 1998). After the breeding season, Sandwich terns migrate south to overwinter in West Africa (Wernham et al., 2002).
Sandwich tern colonies are considered to be highly vulnerable to human disturbance, and colonies may be deserted as a result (Gregersen, 2006; Forrester et al., 2007; Garthe and Flore, 2007; Herrmann et al., 2008; Spaans et al., 2018). However, the response of breeding Sandwich terns to human activity seems to vary considerably among colonies. At the Farne Islands, Sandwich terns have habituated to presence of people on limited footpaths around the perimeter of their colony and continue to incubate when people are no more than 20m away. At many other Sandwich tern colonies where people are not normally present, Sandwich terns will leave their nests and chicks when people approach at much greater distances. Recognising that monitoring numbers and breeding success of Sandwich terns by visiting colonies tends to cause excessive disturbance, Spaans et al. (2018) tested the use of a drone, flown 15-20 m above nesting Sandwich terns at appropriate dates through the breeding season at colonies in The Netherlands, to count breeding numbers and breeding success from photographs. They found that the drone caused “hardly any visible disturbance to the birds” but gave highly accurate data on breeding numbers and breeding success, so was considered much better than using human observations at Sandwich tern colonies. The same conclusion was reached by Valle and Scarton (2021) in Italy.
Away from their colonies, Sandwich terns seem to be at relatively low risk of human disturbance when at sea. Perrow et al. (2011) followed breeding adult Sandwich terns foraging at sea from colonies in north Norfolk over distances of up to 72 km, keeping the boat about 20 to 100m from the bird. They note that “birds generally seemed to ignore the boat”. On the rare occasions (<1% of tracked birds) where birds seemed to respond to the boat, they increased their distance from the bird, and considered that foraging tracks and behaviours were broadly unaffected by their boat following the selected individuals. Sandwich terns will rest on shore at quiet coastal sites, especially during late summer after breeding is completed. This study has been unable to find data on flight initiation distances at such sites, but the locations used by Sandwich terns for post-breeding roosting seem to indicate that they select open areas with low risk of human disturbance (Tierney et al., 2016).
Likely sensitivity to disturbance = High
Quantitative information = No evidence
Breeding season buffer zone ≥ 200m
Sandwich tern is assessed to have a high sensitivity to human disturbance at breeding colonies, although away from breeding grounds, sensitivity is considered to be low.
There are a lack of disturbance studies recording AD/FID values for Sandwich tern. However, non-quantitative studies suggest that buffer zones required to protect Sandwich terns during the breeding season may be similar to those required for other tern species.
In the UK, Sandwich tern has the potential to be disturbed at breeding colonies. From studies on other tern species, it is suggested that buffer zones around breeding colonies should not be less than 200m to protect from pedestrian disturbance.
Knowledge gaps
Lack of studies providing AD/FID values during the breeding season.
Common tern, Sterna hirundo
Conservation Status
UK: Amber List
European: Least Concern, Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 11,000 (8,900-13,500) breeding pairs (Woodward et al., 2020); Scottish population = 4,800 Apparently Occupied Nests, 2,000-20,000 individuals during passage periods (Forrester et al., 2012).
UK long-term trend
Declining breeding distribution in Scotland and Ireland contrasting with gains in eastern and central England; the breeding range has virtually halved in Ireland since 1968-72, whilst in Britain a 13% expansion is apparent (Balmer et al., 2013). Gains in inland England are likely to have resulted from the creation of man-made waterbodies, losses in Scotland and Ireland have been attributed to increases in predation (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Common tern was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in tern colony in North America: Mean FID = 10m (Nisbet, 2000).
Surveyor walking in tern colony in the USA: Range of mean FID = 7.3 to 8.1m (Burger and Gochfeld, 1988).
Surveyor walking towards nesting site in the USA: Mean FID = 142m (n = 18); Min/Max FID = 48 to 400m (Erwin, 1989).
Drone in North America: Min/Max FID = 91 to 122m (n = 502) (Chabot et al., 2015).
Nonbreeding season:
Surveyor walking in a range of habitats in Australia: Mean FID = 20.5m (n = 8) (Weston et al., 2012).
Surveyor walking in Sir Lanka: FID = 66 (n = 1) (Gnanapragasam et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Pedestrian walking/running near a tern colony in a range of locations: Buffer zone = 100 to 400m (Carney and Sydeman, 1999).
Surveyor walking towards nesting site in the USA: Buffer zone = 200m. A buffer zone of 300m may be required to protect colony sites early in the season before birds are established (Erwin, 1989).
Motorised watercraft near a tern colony in a range of locations: Buffer zone = 100m (Carney and Sydeman, 1999).
Motorised watercraft (Jet-ski) in the USA: Buffer zone = 100m (Burger, 1998).
Ecology and non-quantitative disturbance responses
Common tern is a summer visitor to the UK. In Scotland, common tern is primarily a coastal breeding species, the main concentrations are on lochs and islands of the west coast, Outer Hebrides, Northern Isles and the Inner Moray Firth (Balmer et al., 2013). In central and eastern England, breeding common terns are more often located at inland colonies (although there are some coastal colonies such as those in Northumberland) and, in Ireland, colonies are clustered by the coast as well as inland (Balmer et al., 2013). This species breeds on the ground in the open, usually on bare substrate, and makes a shallow scrape on the ground for a nest (Snow and Perrins, 1998). Like other tern species, common terns chiefly feed on marine fish by plunge diving (Snow and Perrins, 1998). After the breeding season, British breeding common terns migrate south to overwinter off the west coast of Africa, principally along the Gulf of Guinea coast between Sierra Leone and Ghana (Wernham et al., 2002).
Common terns may tolerate some forms of human disturbance and are able to habituate to human presence within colonies. Research studies within common tern colonies have shown that even with repeated disturbance, handling and trapping of chicks and adults, breeding success is not significantly reduced (Nisbet, 2000; Galbraith et al., 1999; Morris and Burness, 1992; Burger and Gochfeld, 1991), although removing the first egg may cause some pairs to move to another nest site within the colony (Arnold et al., 1998). Morris and Burness (1992) found that attaching radio transmitters to common terns did not affect nest attendance or chick feeding rates. Nisbet (2000) found that after 30 years of visiting breeding tern colonies, common terns allow approach to within 10m. Chabot et al. (2015) have found that common terns quickly become habituated to the presence of a drone.
However, ecotourists visiting tern colonies that are not habituated to regular human presence may be a cause of disturbance. Erwin (1980) found that common terns were disturbed from preferred nesting sites on barrier beaches in New Jersey by human activity. Common terns nesting in colonies with more exposure to human leisure activity return faster to the colony after banding than terns nesting in more remote colonies (Burger and Gochfeld, 1991; Nisbet, 1981). Erwin (1998) regards a 200m buffer zone (300m early in the season before birds are established) is required to protect common tern colonies from disturbance (people on foot) at colonies in Virginia and New Carolina, although Nisbet (2000), recommends that waterbird colonies should be managed to promote habituation with the presence of wardens or monitors to disturb the colony ‘frequently, regularly and predictably’.
Likely sensitivity to disturbance = Medium/High
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 200-400m
Common tern is assessed to have a medium to high sensitivity to human disturbance at breeding colonies, although away from breeding grounds, sensitivity is likely to be low.
The maximum FID value recorded for common tern is 400m when approached by a pedestrian during the breeding season, although the majority of recorded FID values are under 200m. When approached by a drone during the breeding season, the maximum FID value recorded is 122m. During the breeding season, buffer zones ranging between 100 and 400m have been proposed to protect common terns from pedestrian disturbance and a buffer zone of 100m has been proposed for motorised watercraft disturbance.
In the UK, common tern has the potential to be disturbed at breeding colonies. A buffer zone between 200-400m is suggested to protect common tern colonies from pedestrian disturbance, although a larger buffer zone may be required if terns are not habituated to disturbance or if disturbance occurs early in the breeding season (i.e. during egg laying).
Knowledge gaps
Current studies provide a moderate range of FID values during the breeding season. Future studies should specify habituation to disturbance when recording AD/FID.
Arctic tern, Sterna paradisaea
Conservation Status
UK: Amber List
European: Least Concern, Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 53,500 breeding pairs (Woodward et al., 2020); Scottish population = 47,300 Apparently Occupied Nests, 10,000-200,000 individuals during passage periods (Forrester et al., 2012).
UK long-term trend
UK breeding range shows an overall range contraction of 31% since 1968-72, losses are greatest in western Scotland (especially Northern Isles) which have been attributed to predation (particularly American mink) and food shortages (Balmer et al., 2013). Annual colony sizes fluctuate, a 29% decline in numbers was recorded for Britain and Ireland between 1985/88 – 1998/2002 and a 15% decline during 2000-11, poor productivity and poor recruitment are noted as reasons for the decline (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Arctic tern was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking towards nesting site in Canada: Range of mean FID = 37 to 92m (n = 143); Max FID = 160 (Mallory, 2016).
Aircraft (helicopter) flying over a tern colony in Canada: Mean FID = 1000m (Mallory, 2016).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Surveyor walking towards nesting site in Canada: Buffer zone = 100 to 200m (Mallory, 2016).
Aircraft (helicopter) flying over a tern colony in Canada: Buffer zone = 2000m (Mallory, 2016).
Ecology and non-quantitative disturbance responses
Arctic tern is a summer visitor to the UK where it is a breeding bird at the southern end of the species’ Arctic range. Arctic terns breed predominantly in coastal areas of Scotland and Ireland; in Scotland the highest abundance is recorded in the Northern Isles, Outer Hebrides and northern Scotland (Balmer et al., 2013). There are relatively few colonies in England, some colonies are present along the Northumberland coast, north-east Anglia, Merseyside and one on the Isle of Man, in Wales colonies are restricted to Anglesey and its offshore islands (Balmer et al., 2013). As with other tern species, Arctic terns breed on open bare ground by making a shallow scrape for a nest; they forage on marine fish by plunge diving (Snow and Perrins, 1998). Arctic terns undertake some of the most extensive migration journeys undertaken by any bird; after the breeding season, Arctic terns migrate to Antarctic waters where they spread along food rich areas at the edge of the ice pack (Wernham et al., 2002).
During the UK breeding season, Arctic terns tend to nest in larger colonies than common terns, and also tend to be much more aggressive towards humans that approach their nests, swooping and pecking people on the head. Human disturbance of nesting Arctic terns is therefore less likely to cause problems than human disturbance of common terns, as people tend to be deterred from Arctic tern nesting areas by the birds’ aggression (Bob Furness, pers. obs.). However, there is some evidence to suggest that in a highly disturbed environment, human disturbance can have an effect on Arctic terns. It has been demonstrated on the Isle of May that for Arctic terns, the presence of visitors substantially decreases chick provisioning rates compared to when visitors are not present on the island. The highest level of disturbance was found during the afternoon and evening, when peak chick provisioning occurred (Bogdanova et al., 2014).
Foraging Arctic terns show very little or no behavioural response to the presence of people on the shoreline, so disturbance of foraging or commuting Arctic terns is unlikely. Arctic terns will roost on beaches when not breeding, mostly after the breeding season, and at that time may be displaced from a resting area by human disturbance. However, they are more likely to simply move to a nearby undisturbed area (Bob Furness, pers. obs.).
Likely sensitivity to disturbance = Medium
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone ≥ 200m
Arctic tern is assessed to have a medium sensitivity to human disturbance at breeding colonies, although away from breeding grounds, sensitivity is considered to be low.
The maximum FID value recorded for Arctic tern during the breeding season is 160m when approached by a pedestrian and 1km when approached by a helicopter, although quantitative studies are limited for this species. Buffer zones between 100 and 200m and up to 2km have been suggested to protect Arctic terns from pedestrian disturbance and helicopter disturbance respectively during the breeding season.
In the UK, Arctic tern has the potential to be disturbed at breeding colonies. A minimum buffer zone of 200m is suggested to protect Arctic tern colonies from pedestrian disturbance, although a larger buffer zone may be required if terns are not habituated to disturbance or if there is likely to be aerial disturbance above the colony.
Knowledge gaps
Few studies producing AD/FID values during the breeding season.
Roseate tern, Sterna dougallii
Conservation Status
UK: Red List, Schedule 1
European: Least Concern, Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 100 breeding pairs (Woodward et al., 2020); Scottish population = 4 breeding pairs, 5-20 during spring and autumn passage (Forrester et al., 2012).
UK long-term trend
Eaton et al. (2021) state a stable number of breeding birds (+26%) over 25 years.
Numbers of roseate terns at the UK’s most important roseate tern colony on Coquet Island have continued to grow; the number of breeding adults that were hatched on the island itself has risen steadily from 20% in 2006 to nearly 60% in 2019 (Eaton et al., 2021).
AD/FID
Quantitative disturbance distances
Roseate tern was not included in Ruddock and Whitfield (2007).
Breeding season:
Pedestrian leisure (unspecified) along the shoreline of Cape Cod Peninsula: Mean FID = 115.3m (n = 356), Max FID = 200m (Althouse et al., 2019).
Surveyor walking in tern colony in America: Range of mean FID = 6 to 6.5m (Burger and Gochfeld, 1988).
Nonbreeding season:
Surveyor walking in Africa: FID = 44.0m (n = 1) (Weston et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Pedestrian leisure (unspecified) along the shoreline of Cape Cod Peninsula: Minimum buffer zone = 100m (Althouse et al., 2019).
Pedestrian activity around a tern colony: Buffer zone = 100 to 180m (Carney and Sydeman, 1999).
Ecology and non-quantitative information on disturbance responses
Roseate tern is a summer visitor to the UK where it is a very rare and localised breeder at the coast; inland records are extremely rare for this species (Wernham et al., 2002). The majority (97%) of the UK and Ireland breeding population is located at three colonies including: Coquet Island (northeast England) and Rockabill and Lady’s Island Lake in the east of Ireland; numbers occurring at other colonies are very small, this species occasionally attempts to breed with common terns (Balmer et al., 2013). Roseate terns prefer breeding sites close to clear, shallow sandy fishing grounds; they generally nest under some cover from vegetation or rocks but will also nest on open sand, and will use tern nest boxes which can give added protection from predators, weather and disturbance; birds forage by plunge diving for marine fish (Snow and Perrins, 1998). Roseate terns do not overwinter around the UK, after the breeding season, British birds migrate south to overwinter in coastal Ghana (Wernham et al., 2002; Forrester et al., 2012).
Roseate tern is considered to be a particularly sensitive species to human disturbance. As this species is confined to so few breeding colonies, there is potential for significant disturbance during the breeding season as colonies are vulnerable to localised, stochastic events (OSPAR Commission, 2009). Uncontrolled disturbance to nesting terns (by humans or predators) can lead to abandonment and long-term disuse of sites (Monteiro et al., 1996). In the Azores archipelago, disturbance to wildlife has increased through human recreational activities (fishing, boating, scuba-diving, crab and limpet collecting, picnicking). The largest Azorean colony of roseate terns (200 clutches) was completely abandoned in 1992 after disturbance from picnickers, and in 1990, about 40 eggs were broken by fishermen; in each case, roseate terns did not return to the colony the following year indicating that disturbance may play an important role in colony shifting from year to year (Monteiro et al., 1996). At a stopover site in Cape Cod, Althouse et al. (2019), found that pedestrian activity (particularly activity involving rapid movement such as jogging) caused terns to flush at greater distances compared with shorebirds and gulls, even though gulls are kleptoparasites of terns (although common terns are more commonly targeted in a mixed tern colony). Althouse et al. (2019) suggested that a minimum buffer zone of 100m should be used by managers to protect staging roseate terns, although larger buffer zones may be necessary in areas that are frequented by smaller tern flocks because terns in small flocks may be more sensitive to disturbance than when in larger flocks. Carney and Sydeman (1999) suggested that tern colonies should not be entered within 100 to 180m.
In overwintering grounds in coastal Ghana, roseate terns are vulnerable to trapping by humans for food, sport and sale, the majority of trappings involve first-year birds which affects recruitment into the breeding population (Forrester et al., 2012).
Likely sensitivity to disturbance = High
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone ≥ 200m
Roseate tern is assessed to have a high sensitivity to human disturbance at breeding colonies, particularly because this species is confined to so few breeding colonies.
Quantitative studies are limited for roseate tern, but the maximum FID value recorded for this species when approached by a pedestrian is 200m during the breeding season and 44m during the nonbreeding season. Buffer zones between 100 and 180m have been suggested to protect roseate terns from pedestrian disturbance during the breeding season.
In the UK, roseate tern has the potential to be disturbed at breeding colonies. A minimum buffer zone of 200m is suggested to protect roseate tern colonies from pedestrian disturbance.
Knowledge gaps
Lack of studies providing AD/FID values during the breeding season.
Species tables: Owls
Snowy owl, Bubo scandiacus
Conservation Status
UK: Former breeder, Schedule 1
European: Least Concern
UK status
Accidental, Former Breeder
UK and Scottish population estimate
Scottish population = 1 breeding pair annually 1967-75 (Forrester et al., 2012). No known breeding attempts since 2001 in Ireland (Balmer et al., 2013).
UK long-term trend
Small but fluctuating numbers occur. Five different individuals were on St Kilda in May-August 2007 (Miles and Money, 2008). Two nonbreeding mobile birds were recorded during the summer (one in the Outer Hebrides, the other in the Channel Islands) between 2008 and 11; six or seven mobile birds were present during the winters between 2007 and 11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Snowy owl was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor approaching a nest site on Baffin Island, Canada: Min/Max FID (of brooding female) = 274.3 to 548.6m. (Watson, 1957).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone available for snowy owl.
Ecology and non-quantitative information on disturbance responses
Snowy owl is a rare winter migrant to Scotland. This species has a circumpolar breeding distribution on the high-arctic tundra, migrants in Scotland may originate from the European Arctic (Balmer et al., 2013). One pair bred in Fetlar in Shetland between 1967-75, but successful breeding attempts ceased when the breeding male died during the winter of 1975/76 (Balmer et al., 2013). Snowy owl is a ground nesting species, the nest is usually a shallow scrape on a raised bit of ground above the snow (Snow and Perrins, 1998). In a study in Norway, Solheim et al. (2021) suggested that male snowy owls selected elevated mounds, rocks or heights around the nest site in order to have the best view of the territory and keep a look out for prey and potential threats. A wide distribution of a small number of overwintering birds (6-7 individuals) was recorded in the UK between 2007 and 11, mainly in the Outer Hebrides, Orkney, Scottish Highlands, Channel Islands and Western Ireland (Balmer et al., 2013). Snowy owls feed on small mammals and medium sized birds (lemmings or voles on tundra), foraging may take place during the day although most hunting is carried out in the twilight of morning or evening (Snow and Perrins, 1998). Nonbreeding birds at St Kilda in summer 2007 fed on mice, adult puffins and skua chicks (Miles and Money, 2008). Outside the breeding season, snowy owls are solitary birds, in over wintering areas they are often seen resting on the ground or on mounds, rocks and fences.
Due to their remote breeding grounds, breeding snowy owls are largely free from direct human disturbance. However, snowy owls that are disturbed by pedestrians and predators on breeding grounds will strongly defend their nest sites and this species is known to attack people as well as Arctic foxes and dogs that come too close (Wiklund and Stigh, 1983; Watson, 1957; Sutton and Parmelee 1956). On Baffin Island in Canada, Watson (1957) noted that surveyors could not approach a nest without being seen by the male snowy owl and he described the attack as “silent and unexpected”; the owls would sometimes beat their wings on the surveyor’s head and give a painful blow with the back of their feet, sometimes with claws extended. Sutton and Parmelee (1956) also report being struck by the talons of snowy owls on Baffin Island, but also note that some warning of an attack is given; owls would hoot from a distant hilltop or while flying from one lookout post to another. Wiklund and Stigh, (1983) noted that as soon as an intruder faced an approaching snowy owl, the owl generally interrupted the attack even when only 5-10m from the intruder. Sutton and Parmelee (1956) found that snowy owls would not attack until the surveyors were within 100 yards (ca 91.5m) of a nest or young. Watson (1957) recorded at one location on Baffin Island that brooding female snowy owls flew away from surveyors at 300 yards (ca 274.3m), alighting 500 yards (ca 457m) beyond, and the male came no nearer than 50 yards (ca 46m), although when the surveyor moved 300 yards away from the nest the female returned at once, while the male watched from a perch. At another location, Watson (1957) noted that the owls were a bit shyer and brooding females would fly when the surveyor was 600 yards (ca 548.6m) away and the males would not come closer than 200 yards (ca 183m), though the nests contained young.
In Norway, studies on snowy owls have suggested that this species is potentially sensitive to a wide range of human disturbance, sources of pedestrian disturbance may include: tourism, recreation, reindeer husbandry, motorised traffic, dogs, photographers, ornithologists and scientists (Heggøy and Øien, 2014). Other human related disturbance including: egg collection, illegal hunting (still legal hunting in Alaska), environmental contaminants (PCBs, POPs) and collisions (cars, aeroplanes and power lines) are also considered potential threats (Heggøy and Øien, 2014).
On the Outer Hebrides, flushing distances to human disturbance have been found to be quite variable as snowy owls often sit in open machair grassland areas where people can be visible at long distances, however, birds can often be approached quite closely (c. 10m) without flushing if the approach is done slowly and sensitively, although birds will flush if birdwatchers/tourists approach too closely or surround an individual (Andrew Stevenson, pers. comm.) Snowy owls can be flushed by crofting/farming activities as well, although these sorts of regular activities are often ignored by individual birds, especially if the activity is at a distance (Andrew Stevenson, pers. comm.) On St Kilda, the current resident snowy owl has habituated to some degree to human presence, although this bird will avoid the village on the island where human activity is highest (Andrew Stevenson, pers. comm.). In New Hampshire, pedestrians wishing to approach migrant snowy owls during the nonbreeding season are advised to keep at least 100 feet (ca 30.5m) away from birds on the ground, as at this distance snowy owls may stare at a human present and any closer may cause birds to flush (New Hampshire Audubon, 2021). New Hampshire guidelines state that “flushed birds have collided with stationary objects and once airborne they attract the attention of crows, gulls and hawks, which will pursue and harass them, reducing opportunities to hunt” (New Hampshire Audubon, 2021)
In a study in Norway, Solheim, (2021) found that nonbreeding male snowy owls would approach and attack a vole lure on a line that was pulled by a surveyor who was sitting on the ground or in a car ca 100-500m away, the two female owls included in the study did not show any detectable reaction to the lures. The authors also noted that snowy owls perched 100m or closer to the road; surveyors usually watched the owls from a car to prevent disturbing the birds (Solheim, 2021).
Hardey et al. (2013) recommend that snowy owls should not be disturbed during laying or incubation, the authors also recommend that due to the rarity of this species within Britain and Ireland, all observations on the breeding snowy owl should be made from a distance, unless licenced surveyors have a specific need to collect information on clutch or brood size.
Likely sensitivity to disturbance = Medium
Quantitative information = Low agreement & Limited evidence
Nonbreeding season buffer zone = 150-500m
Snowy owl is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for snowy owl; the maximum FID value recorded for this species is 548.6m when approached by a pedestrian in Canada during the breeding season, but as this species does not breed in the UK, quantitative values recorded during the breeding season may not be relevant to disturbance in the UK. There are no records of AD/FID values for pedestrian disturbance during the nonbreeding seasons, but Solheim, (2021) indicates that snowy owls may approach people within 100-500m.
In the UK, snowy owl is most likely to be disturbed on foraging and roosting grounds during the nonbreeding season. There are no published protection buffer zones for snowy owls, but from non-quantitative studies as well as studies on other owl species, a minimum buffer zone of 150-500m is suggested to protect foraging and roosting snowy owls during the nonbreeding season from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions.
Knowledge gaps
Lack of British studies measuring AD/FID for a range of pedestrian disturbance activities.
Long-eared owl, Asio otus
Conservation Status
UK: Green List
European: Least Concern
UK status
Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 1,800-6,000 breeding pairs (Woodward et al., 2020); Scottish population = 600-2,200 breeding pairs, 2,000-12,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
Census methods do not provide accurate population estimates for this elusive and cryptic species (Forrester et al., 2012; Balmer et al., 2013), so trends in numbers are uncertain. However, while numbers may have declined in Scotland and England, they seem to have increased in Ireland between 1968 and 72 and 2007-11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
FID update (Díaz et al., 2021) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Spain: FID = 12m (n = 1) (Díaz et al., 2021).
Surveyor approaching a nest in Italy: Min/Max FID = 3 to 42.5m (Galeotti et al., 2000).
Surveyor walking in a forest habitat in the USA: Min/Max FID = c.3 to 8m (Wilson, 1938).
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD = 30m (n = 5 to 6); Min/Max AD (80% opinion range) = <10 to 300m; Min/Max AD (90% opinion range) = 150 to 300m.
Range of median FID = 5 to 30m (n = 5 to 7); Min/Max FID (80% opinion range) = <10 to 300m
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone updates published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Disturbance free zone = 75 to 125m (Petty, 1998).
Construction work in California: Exclusion zone = 150m (Ruddock and Whitfield, 2007).
Ecology and non-quantitative information on disturbance responses
Long-eared owl is a solitary and territorial resident breeder in the UK with a habitat preference for open spaces with coniferous and scrub habitats containing abundant prey; this species will also breed in deciduous woodland (Balmer et al., 2013; Snow and Perrins, 1998). Breeding locations are widespread and scattered across Britain and Ireland, although long-eared owls are relatively uncommon in Scotland and England (Balmer et al., 2013). In Ireland it is the most abundant owl species and probably benefits from the absence of competing dominant tawny owls; in England, numbers are highest in northern areas with declines in the southeast and Wales (Balmer et al., 2013). In Scotland, long-eared owls are predominantly present in the south, east and north-east (except for the Black Isle where numbers are declining) and are absent from the north-west and the Northern Isles, except for a few pairs on the Inner and Outer Hebrides (Balmer et al., 2013; Forrester et al., 2012). Long-eared owls generally nest in trees; this species doesn’t build its own nest but reuses old nests of other species, principally crows, sparrowhawks and magpies; nest boxes will also be used (Forrester et al., 2012; Snow and Perrins, 1998). Some long-eared owls may not lay eggs after establishing a nesting territory and separating early breeding failure from genuine non-breeding is particularly difficult for this species (Hardey et al., 2013). The diet of long-eared owl is mainly small rodents, especially voles, but other prey items may also include some birds, larger mammals and shrews; the diet is often more diverse in summer (Snow and Perrins, 1998).
During the nonbreeding season, resident breeding long-eared owls are joined by migrants from Fennoscandia, Russia and elsewhere in eastern Europe; there are fewer movements between eastern Britain and the Low Countries (Wernham et al., 2002). British breeders are fairly sedentary, although male birds may remain further north than females in some parts of the range (Wernham et al., 2002), but generally distributions between breeding and nonbreeding seasons are fairly similar (Balmer et al., 2013). In winter, communal roosts form, often in scrub near water and always in proximity to open habitat suitable for hunting (Wernham et al., 2002).
Long-eared owls are highly cryptic in woodland, very secretive and difficult to find which makes this a problematic species to survey and may provide some protection against some sources of human disturbance. Nesting birds vary in their behaviour towards intruding people. At the approach of a human, most remain tight on the nest to within a few metres (Galeotti et al., 2000), a few fly to deeper cover, and a few will swoop at people or perform a distraction display a few metres away (Cramp, 1985). In a study in Italy, Galeotti et al. (2000) found that nest defence increased significantly throughout the breeding season because older chicks were defended more strongly than younger chicks and eggs; median flushing distances of females occurred in the range of 3-42.5m from the start of incubation to early fledging. In a study in the USA on breeding owls, Wilson (1938) recorded that once disturbed by a surveyor, long-eared owls would flush at distances of c.3-8m and land again c.22-90m away.
Whilst long-eared owls are mostly found in woodland in the UK, in eastern Europe this species often occurs in urban habitats, both for breeding and for communal roosting. In urban habitats, long-eared owls may apparently be highly tolerant of human activity and they are thought to benefit from milder microclimates in urban roosts as well as reduced predation risk and availability of urban bird prey (Makarova and Sharikov, 2015; Mérö and Žuljević, 2020; Mak et al., 2021). Pirovano et al. (2000) found that long-eared owls adapt well to urban environments in the winter, in a study in Italy the authors observed urban roosts of up to 75 birds in public parks and private gardens.
However, long-eared owls can be sensitive to disturbance, particularly early in the nesting cycle and at communal roosts (Hardey et al., 2013). Hardey et al. (2013) recommend that any disturbance of potential roost sites by surveyors should be carried out as close to dusk as possible so that birds are not forced to leave roosts for long periods during daylight.
Likely sensitivity to disturbance = Medium
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone = 100-300m
Nonbreeding season buffer zone = 100-300m
Long-eared owl is assessed to have a medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for long-eared owl, but the maximum FID value recorded for this species is 42.5m when approached by a pedestrian during the breeding season; there are no records of AD/FID values during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for long-eared owl during the breeding season is 150 to 300m.
Buffer zones range from 75 to 125m to protect long-eared owls from forestry operations during the breeding season in the UK. An exclusion zone of 150m around nest sites has been recommended for construction activity in the USA.
In the UK, long-eared owl is most likely to be disturbed at nest sites early on in the breeding season as well as at communal roosting areas during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 100-300m is suggested to protect both breeding and nonbreeding long-eared owl from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions, especially during the nonbreeding season. A buffer zone at the lower end of this range, or even lower may be sufficient to protect individuals that have some habituation to human presence.
Knowledge gaps
Lack of British studies measuring AD/FID for a range of pedestrian disturbance activities.
Short-eared owl, Asio flammeus
Conservation Status
UK: Amber List, Schedule 1
European: Least Concern, Annex 1
UK status
Migrant/Resident Breeder, Passage/Winter Visitor
UK and Scottish population estimate
UK population = 620-2,200 breeding pairs (Woodward et al., 2020); Scottish population = 125-1,250 breeding pairs, 300-3,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
Balmer et al. (2013) note widespread declines in numbers in Britain and Ireland, as also found in continental Europe. Declines have occurred in Scotland (Forrester et al., 2012) which most likely relate to maturing of plantation forestry so loss of nesting habitat in young plantations.
AD/FID
Quantitative disturbance distances
FID update (Booms et al., 2010) published since Ruddock and Whitfield 2007.
Breeding season:
Aircraft (helicopter) in Alaska: Mean FID = 55m, Min/Max FID = 50 to 60m.
(Booms et al., 2010).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 75 to 125m (n = 13 to 12); Min/Max AD (80% opinion range) = <10 to 500m; Min/Max AD (90% opinion range) = 300 to 500m.
Range of median FID = 5 to 75 m (n = 14); Min/Max FID (80% opinion range) = <10 to 500m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone updates published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Safe working distance = 300 to 600m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 275 to 325m (Petty, 1998).
Ecology and non-quantitative information on disturbance responses
Short-eared owl is a resident breeder and migrant species in the UK where it mainly inhabits areas of open country in Scotland and northern England (Balmer et al., 2013). Numbers are highest on Orkney, Outer Hebrides (Uists) and in the Pennines, elsewhere numbers of breeding birds are widely scattered and involve a small number of pairs in lowland coastal marshes and extensive grassland (Balmer et al., 2013). Short-eared owls have a habitat preference for upland heather grass-heather moorland, rough grassland, bogs and young forestry plantations populated with small mammal prey, particularly field voles (Balmer et al., 2013; Forrester et al., 2012). Arable areas are little used for breeding as are re-stocked conifer forests (Forrester et al., 2012). This species nests and often roosts on the ground; the nest is a shallow scrape roughly lined with pieces of vegetation in amongst the thick cover of grass, reeds and heather etc (Snow and Perrins, 1998).
In the nonbreeding season, some British breeding short-eared owls migrate to southern Europe while others remain in the UK but move from uplands to coastal marshes, dunes and farmland; birds remaining in the UK are joined by Fennoscandian breeders (Balmer et al., 2013;. Wernham et al., 2002). Overwintering birds can be found along the British east coast from Fife to Kent as well as around large river valleys and lowlands of England; birds breeding in Orkney, the Uists and the Pennines overwinter close to their breeding grounds (Balmer et al., 2013). In winter, short-eared owls generally roost communally, regularly on the ground at favoured locations in amongst vegetation (Wernham et al., 2002). Roosts can hold a dozen owls or more, but due to the mobility of the population in winter, there can be a high turn-over of numbers at roost sites (Wernham et al., 2002).
Fernandez-Bellon et al. (2021) reviewed the threats to short-eared owls and identified ecological factors (particularly prey availability, but also predation and extreme weather), changes in land use (habitat loss and agricultural intensification), persecution (shooting), and accidental nest destruction resulting from agricultural practices, as significant threats. They did not identify human disturbance as a threat. Forrester et al. (2007) identify habitat loss and illegal persecution as threats in Scotland, but did not indicate human disturbance to be a factor, although they note that short-eared owl roosts tend to be in remote locations away from human activity.
Van Gompel (1979) identified human disturbance as a major cause of displacement and abandonment of roost sites of short-eared owls wintering on the Belgian coast, though part of that related to illegal hunting of the species in Belgium. Cramp (1985) notes that short-eared owls are “wary”, but “not markedly shy”. However, Cramp (1985) states that birds in winter roosts tend to fly when a person approaches within ca.50m of a roost site, although such birds “rarely fly far before alighting”. Human disturbance near the nest normally results in the female sitting tight, often only flushing off the nest when almost stepped on (Cramp, 1985). Adults, mostly males, will sometimes attack people that approach the nest, sometimes use a distraction display, and sometimes alternate between these behaviours (Cramp, 1985). Reaction distance of males to humans increases when there are chicks in the nest, but typically the male may attack a person when they approach within 200m of the nest, barking in agitation and swooping towards the person, not normally making contact, but in some cases hitting and even drawing blood (Cramp, 1985).
Hardey et al. (2013) suggest that short-eared owls are potentially sensitive to disturbance during the breeding season, the authors recommend that the nests of this species should not be visited in cold, wet weather. Hardey et al. (2013) also recommend that vantage points for viewing short-eared owls are situated at least 500m away from areas of activity / nests to minimise the risk of disturbance and that searches for roost sites should be avoided due to the disturbance that this causes.
Likely sensitivity to disturbance = Medium/High
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone = 300-500m
Nonbreeding season buffer zone = 300-500m
Short-eared owl is assessed to have a medium to high sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for short-eared owl; the maximum FID value recorded for this species is 60m when approached by a helicopter in Alaska during the breeding season. There are no records of AD/FID values for pedestrian disturbance during either the breeding or nonbreeding seasons, but Cramp (1985) indicates that pedestrian disturbance may have an FID value within c.50m. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for short-eared owl during the breeding season is 300 to 500m.
Buffer zones range from 275 to 600m to protect short-eared owls from forestry operations during the breeding season in the UK.
In the UK, short-eared owl is most likely to be disturbed at nest sites in the breeding season as well as at communal roosting areas during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 300-500m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect both breeding and nonbreeding short-eared owls from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence. Forestry operations may require a larger buffer zone up to 600m to avoid disturbance during the breeding period.
Knowledge gaps
Lack of studies measuring AD/FID for a range of pedestrian disturbance activities.
Tawny owl, Strix aluco
Conservation Status
UK: Amber List
European: Least Concern
UK status
Resident Breeder
UK and Scottish population estimate
UK population = 50,000 breeding pairs (Woodward et al., 2020); Scottish population = 6,000 breeding pairs, 12,000 individuals plus ‘floaters’ in winter (Forrester et al., 2012).
UK long-term trend
Atlas survey methods are not very good for tawny owl, and trends in numbers are uncertain. Balmer et al. (2013) suggest increases in north and west Scotland between 1968-72 and 2008-11. Forrester et al. (2012) predict an increase in tawny owl numbers in Scotland as new native woodlands develop and increasing areas of plantation conifer forests reach maturity.
AD/FID
Quantitative disturbance distances
Tawny owl was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat: FID = 26.1m (n = 1) (Díaz et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Forestry operations in the UK: Disturbance free zone = 75 to 125m (Petty, 1998).
Breeding season (Barred owl, Strix variata, stand in species for tawny owl):
Forestry operations in Ontario: Buffer zone = 200m (Naylor, 2009).
Ecology and non-quantitative information on disturbance responses
Tawny owl is a widespread, common resident breeding species in deciduous and mixed woodlands throughout Britain (Balmer et al., 2013). Tawny owls will also inhabit tree-dotted farmland, urban parks and orchards, and even large gardens (Snow and Perrins, 1998). This species is absent from treeless areas including the Northern Isles, Outer Hebrides, some Inner Hebridean islands, Isles of Scilly and open areas of northern Scotland, it is also absent in the Channel Islands and Ireland (Balmer et al., 2013). Tawny owl is generally a hole nesting species, selecting holes usually up to 12m above ground (although they can be up to 25m above ground), they will readily take to using nest boxes; this species will also nest on cliffs or buildings often in old magpie nests or occasionally squirrel dreys (Snow and Perrins, 1998). Compared with other owls, tawny owls have a fairly wide diet depending on location. In woodland the diet is mainly rodents (but also birds, amphibians, shrews, earthworms and beetles), in towns, mainly birds are eaten, although also small rodents and other prey as available (Snow and Perrins, 1998). Tawny owls are highly sedentary and show a high degree of site fidelity, birds rarely move more than a few kilometres from their natal sites throughout their lives (Wernham et al., 2002); breeding and nonbreeding distributions are very similar (Balmer et al., 2013). Tawny owl is a solitary species and individuals remain alone or in their pairs throughout the year.
Forrester et al. (2007) did not suggest that human disturbance represented a significant threat to tawny owls in Scotland, their range of habitats brings them into close contact with people, especially in urban environments. While they appear to be tolerant of human activity, van der Horst et al. (2019) attributed lower densities of tawny owl territories close to main roads due to a combination of collision mortality and disturbance of owls by vehicle traffic. When disturbed at the nest, tawny owls vary considerably in terms of behaviour. Females guard the nest, and most go silently into cover if disturbed by a human at the nest, but a few individuals will attack, especially birds in urban habitats where they experience more human disturbance (Cramp, 1985). The most aggressive individuals may attack a person when they come within 50m of a nest containing young, usually swooping from behind and in extreme cases making physical contact and drawing blood (Cramp, 1985). Sacchi et al. (2004) found that tawny owls in urban parkland preferred nest boxes that were more than 6m above the ground, and suggest that this is part of a protection strategy against human disturbance. Frohlich and Ciach (2018) found that urban areas with high levels of human noise at night held lower densities of tawny owls. They suggest that tawny owl hunting efficiency may be reduced in noisy environments, indicating that human noise may be a stronger influence on tawny owls than visual disturbance.
Likely sensitivity to disturbance = Low/Medium
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 50-200m
Nonbreeding season buffer zone ≥50m
Tawny owl is assessed to have low to medium sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for tawny owl; the maximum FID value recorded for this species is 26m when approached by a pedestrian during the breeding season; there are no records of AD/FID values for pedestrian disturbance during the nonbreeding season. Cramp (1985) indicate that pedestrians shouldn’t approach nests any closer than c.50m. Buffer zones range from 75 to 125m to protect tawny owls from forestry operations during the breeding season in the UK.
In the UK, tawny owl is most likely to be disturbed at nest sites in the breeding season, but there is also potential for disturbance at roosting and foraging areas during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 50-200m is suggested to protect nesting tawny owls and a buffer zone of ≥50m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence.
Knowledge gaps
Lack of studies measuring AD/FID for a range of pedestrian disturbance activities. Lack of MAD/buffer zones for tawny owl.
Barn owl, Tyto alba
Conservation Status
UK: Green List, Schedule 1
European: Least Concern
UK status
Resident Breeder
UK and Scottish population estimate
UK population = 4,000-14,000 breeding pairs (Woodward et al., 2020);
Scottish population = 500-1,000 breeding pairs (Challis et al., 2020; Forrester et al., 2012), 1,000-2,000 individuals in winter (Forrester et al., 2012).
UK long-term trend
According to Balmer et al. (2013), barn owls declined from the mid-19th century to the present, owing to changes in agriculture, loss of nest sites, and road traffic collision mortality. However, milder winters, nest box provision and agri-environment schemes may have mitigated that decline in recent years. Atlas maps show a large increase in barn owl distribution in Britain and Ireland between 1968-72 and 2007-11. Forrester et al. (2012) note that the Scottish population has been steadily growing since the 1980s.
AD/FID
Quantitative disturbance distances
No AD/FID updates published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a forest habitat in the USA: Min/Max FID = c.1.5 to 30m (Wilson, 1938).
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD = 5m (n = 10 to 11); Min/Max AD (80% opinion range) = <10 to 100m; Min/Max AD (90% opinion range) = 50 to 100m.
Median FID = 5m (n = 11); Min/Max FID (80% opinion range) = <10 to 100m
(Ruddock and Whitfield 2007; Whitfield et al., 2008a).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (Shawyer, 2011) published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian walking/running in the UK: Buffer zone = 10 to 20m
Artificial lighting in the UK: Buffer zone = 20 to 30m
Motorised vehicle (general) in the UK: Buffer zone = 30 to 40m
Light commercial vehicle/machine (construction activity) in the UK: Buffer zone = 40 to 60m
Heavy commercial vehicle/machine (construction activity) in the UK: Buffer zone = 150 to 175m (Shawyer, 2011).
Forestry operations in the UK: Safe working distance = 100 to 250m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Forestry operations in the UK: Disturbance free zone = 75 to 125m (Petty, 1998).
Ecology and non-quantitative information on disturbance responses
Barn owl is a resident breeding species in the UK. This species is widespread across Britain, but these owls avoid high-altitude and urban areas and are absent from remote islands including the Outer Hebrides and Northern Isles; distribution is patchy in Ireland (Balmer et al., 2013). Barn owls can exploit a wide range of habitats, but they prefer lowlands with trees, especially farmlands with a combination of trees, hedges and aquatic areas with some rough grasslands where mice and other prey can be hunted in low flight (Snow and Perrins, 1998). Barn owl is a cavity nesting species using holes in trees, buildings, cliffs, quarries or rocky outcrops; nests are reused for successive broods and in successive years (Snow and Perrins, 1998). The diet is made up of small mammals, mostly mice and voles, some shrews and also some small birds and amphibians are eaten (Snow and Perrins, 1998). Adult barn owls are sedentary, but juveniles will disperse a median distance of 12km away from their natal sites in the first few weeks after fledging (Wernham et al., 2002); breeding and nonbreeding distributions are very similar (Balmer et al., 2013). Barn owl is a solitary species and individuals remain alone or in their pairs throughout the year.
As the name indicates, barn owls frequently nest in farm buildings, but will also use nest boxes or natural holes in trees. When nesting, barn owls tend to sit tight when a person approaches the nest, even when they come very close (Cramp, 1985). Although eggs may be deserted due to disturbance, barn owl chicks and adults can be ringed at the nest with almost no risk of adults deserting the nest due to the disturbance (Arthur French, pers. Comm.). Barn owls that are hunting show very little avoidance of people or of vehicles. Collision with road traffic is a major cause of mortality in barn owls (Forrester et al., 2007; de Jong et al., 2018).
Barn owls can be sensitive to disturbance at the nest site, particularly early in the nesting cycle. Hardey et al. (2013) recommend that licenced surveyors should take special care to avoid disturbance during pre-laying through to hatching, although the authors also state that nest inspections should not have a detrimental effect if carried out carefully. Hardey et al. (2013) also recommend that barn owls should not be flushed from nests or roosts in daylight because they may be mobbed by other birds and will be reluctant to return, which may affect their survival, particularly in the winter months. In a study in the USA on breeding owls, Wilson, (1938) recorded that once disturbed by a surveyor, barn owls would flush at distances of c.1.5-30m and land again c.90-150m away.
Likely sensitivity to disturbance = Low
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 50-100m
Nonbreeding season buffer zone ≥50m
Barn owl is assessed to have a relatively low sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for barn owl; the maximum FID value recorded for this species is 30m when approached by a pedestrian during the breeding season; there are no records of AD/FID values for pedestrian disturbance during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for barn owl during the breeding season is 50 to 100m, although the authors state that, as barn owl frequently nest in nest boxes ‘overly prescriptive ‘exclusion zones’ based on the upper limits of apparent signs of disturbance in some pairs or situations may not be an appropriate management option in several situations’.
Buffer zones range from 75 to 250m to protect barn owls from forestry operations during the breeding season in the UK. The Wildlife Conservation Partnership guidance recommends buffer zones of 10-20m to protect barn owl from pedestrian disturbance and buffer zones from 20-175m to protect against a range of other disturbances.
In the UK, barn owl is most likely to be disturbed at nest sites in the breeding season, but there is also potential for disturbance at roosting and foraging areas during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 50-100m (considered to be the upper disturbance limit estimated by expert opinion (Ruddock and Whitfield, 2007)) is suggested to protect nesting barn owls and a buffer zone of ≥50m is suggested to protect roosting and foraging birds during the nonbreeding season from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions. A buffer zone at the lower end of this range may be sufficient to protect individuals that have some habituation to human presence. Forestry operations may require a wider buffer zone up to 250m to avoid disturbance during the breeding period.
Knowledge gaps
Lack of studies measuring AD/FID for a range of human disturbance activities.
Species: Other species
Corncrake, Crex crex
Conservation Status
UK: Red List, Schedule 1
European: Least Concern, Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 1,000 territorial breeding males mostly in Scotland (Woodward et al., 2020). Scottish population estimate has increased since Forrester et al. (2012) estimated a population of 1,060 breeding pairs in 2004, 0-10 birds in passage.
UK long-term trend
Eaton et al. (2021) state a strong increase in breeding birds (+108%) over 25 years.
Once an abundant and widespread breeding bird in the UK, there has been a long-term population decline since 1968/72 (Balmer et al. 2013). However, the British range increased by 14% between 1988/91 and 2008/11 and the population increased by 141% between 1993 and 2009, although there have been continued losses in Ireland (Balmer et al. 2013). Gains are largely a result of conservation measures, agri-environmental schemes and a reintroduction programme in eastern England (Balmer et al. 2013).
AD/FID
Quantitative disturbance distances
Corncrake was not included in Ruddock and Whitfield (2007).
Nonbreeding season:
Pedestrian walking/running at a stopover site in Egypt: Mean FID = 2.8m (Eason et al., 2010).
MAD and/or
Buffer zone
Quantitative distances
Breeding season:
Pedestrian (bird monitoring methods in the UK): MAD = 100m (not necessary to approach closer than 100m to pinpoint singing male) (Gilbert et al.,1998).
Ecology and non-quantitative information on disturbance responses
Corncrakes are summer visitors to the UK. The breeding population of corncrake is now mainly confined to a small number of coastal and island strongholds in Scotland and Ireland; the main breeding concentrations are in the Outer and Inner Hebrides with smaller numbers in Orkney, Shetland and coastal areas of Co. Donegal and West Connaught (Balmer et al., 2013). A growing breeding population is also present in the Nene Washes in eastern England where this species was introduced in 2002; a small number of passage birds moving to breeding grounds are also regularly recorded in eastern areas of Scotland and England (Balmer et al., 2013). Corncrakes prefer habitats that are composed of cool, moist stands of grass or herbage (including machair and fields of clover and cereals) that are tall enough to provide concealment; a nest is formed out of dead leaves on the ground concealed by vegetation (Snow and Perrins, 1998). Corncrakes are omnivorous feeding mainly on invertebrates, but small amounts of plant material, especially seeds, are also eaten (Snow and Perrins, 1998). Although there are historical records of corncrakes wintering in the UK, this species is largely migratory; after the breeding season, corncrakes migrate south through France crossing into Africa via Morocco to overwinter in trans-Saharan Africa (Wernham et al., 2002).
Isolated corncrake populations may be vulnerable to disturbance from birdwatchers, but in general this species is not thought to be very sensitive to human disturbance (RSPB, 1996). The decline in corncrake numbers was first noticed in the middle of the 19th century (Balmer et al. 2013; Cocker and Mabey, 2005), but even up to the late 1960s this species was an abundant and widespread breeding bird in the UK. Corncrakes were unable to adapt to changes in land management practices that followed agricultural intensification, particularly the changes that led to the motorisation and early mowing of grass crops for silage which kill their young (Balmer et al. 2013). Conservation measures brought about by the RSPB and adopted into agri-environmental schemes to delay mowing until August and to mow fields from the centre outwards to allow chicks to escape (these methods are referred to as Corncrake Friendly Mowing, CFM) have resulted in recent gains in corncrake numbers (RSPB, 2021b; Balmer et al., 2013; O’Brien et al., 2006).
Despite being a rather timid and highly cryptic species, more often heard than seen, corncrakes are able to tolerate human presence; this species inhabits agricultural areas and will live in close proximity to human activity. For example, in the UK, corncrakes have been reported to call within close proximity to human habitation (e.g. Norris, 1945; Cocker and Mabey, 2005) and the number of corncrakes recorded in a Moscow city park reportedly remained stable between 1928 and 1994 despite heavy recreational pressure (summarised in RSPB, 1996). Some corncrakes are able to habituate to human presence to such an extent that they will visit human dwellings to be fed (Cocker and Mabey, 2005).
However, the small, isolated populations that are now present in the UK are more likely to be impacted by disturbance than a widespread species (RSPB, 1996). In 2014, a male corncrake was heard calling for the first time in 15 years on Rathlin Island in Northern Ireland, but it is thought that this bird left the island due to disturbance caused by a helicopter landing briefly in an uncropped hayfield where the corncrake had been calling (RSPB, 2014).
Likely sensitivity to disturbance = Medium
Quantitative information = Low agreement & Limited evidence
Breeding season buffer zone ≥100m
Corncrake is assessed to have a medium sensitivity to human disturbance; the sensitivity of this species has increased as breeding populations have become more isolated.
Quantitative studies measuring AD/FID are very limited for corncrake; the maximum FID value recorded for this species when approached by a pedestrian is 2.8m during the nonbreeding season. A MAD of 100m has been recommended to protect corncrakes from pedestrian disturbance during the breeding season.
In the UK, corncrake has the potential to be disturbed on breeding grounds. Depending on the level of habituation to disturbance, a buffer zone of at least 100m is suggested to protect breeding corncrake from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions.
Knowledge gaps
Lak of any AD/FID studies during the breeding season.
European nightjar, Caprimulgus europaeus
Conservation Status
UK: Amber List
European: Least Concern, Annex 1
UK status
Migrant Breeder, Passage Visitor
UK and Scottish population estimate
UK population = 4,600 (3,700-5,500) territorial breeding males (Woodward et al., 2020); Scottish population = 27 territorial males, 1 record in winter, 0-4 during spring and autumn passage (Forrester et al., 2012).
UK long-term trend
Historically a widespread breeding species in the UK, the range contracted by 51% and 88% in Britain and Ireland respectively between 1968/72 and 1988/91 (Balmer et al., 2013). However, since this time the British breeding population doubled from 2,100 territorial males in 1981 to 4,600 in 2004, the breeding range also expanded by 18% between 1988/91 and 2008/11 (Balmer et al., 2013; Woodward et al., 2020).
AD/FID
Quantitative disturbance distances
FID update (Dolman, 2010) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a forest habitat in England: Mean FID = 10m (n = 22) (Dolman, 2010).
Pedestrian walking/running, disturbance estimated by expert opinion:
Range of median AD = 5 to 18m (n = 12); Min/Max AD (80% opinion range) = <10 to 150m; Min/Max AD (90% opinion range) = 100 to 150m.
Median FID = 5m (n = 14); Min/Max FID (80% opinion range) = <10 to100m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
MAD and/or
Buffer zone
Quantitative distances
Buffer zone update (Langston et al., 2007) published since Ruddock and Whitfield (2007).
Breeding season:
Pedestrian leisure activity (general) on a heathland habitat in England: Buffer zone = 150m (Langston et al., 2007)
Pedestrian leisure activity (general) on a heathland habitat in England: Buffer zone = 500m (Murison, 2002).
Forestry operations in the UK: Safe working distance = 50 to 200m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Ecology and non-quantitative information on disturbance responses
Nightjars are summer visitors to the UK. Only a small proportion of the European population breeds in the UK, the majority of birds breed in Spain (Wernham et al., 2002). Nightjars in Britain are widely distributed across England and Wales, the highest concentrations are in East Anglia and southern England (Balmer et al., 2013). Although historically nightjar was once widespread in Scotland (as they were throughout the UK), this is now a very scarce breeding species mostly confined to the south-western area of Dumfries and Galloway (Balmer et al., 2013) in clearings within conifer plantations (Forrester et al., 2012). This is a nocturnal species which feeds on flying insects, mostly moths and beetles (Snow and Perrins, 1998). The preferred habitat of nightjars in the UK includes lowland heathland and felled or recently planted conifer plantations, though coastal moorland (Cornwall), sweet chestnut coppice (Kent) and sand dunes (Suffolk) may also be occupied (Balmer et al., 2013). Nightjars make a shallow scrape on the ground for a nest which may be located in the open, in woodland clearings or in amongst scrub and tall vegetation (Snow and Perrins, 1998). . Some suitable habitat is available in Scotland in the form of young conifer plantations, but the lowland dry heaths generally associated with this species in England are rare in Scotland (Forrester et al., 2012). Nightjars do not overwinter in the UK, after the breeding season, this species migrates south to overwinter in eastern and southern Africa (Wernham et al., 2002).
Nightjars are highly cryptic in woodland, secretive and difficult to find. Their camouflage may provide protection against some sources of human disturbance (e.g. some pedestrians and predators) and birds will often sit unseen on the ground at their roost or nest site until approached within a few metres (Wernham et al., 2002). Ruddock and Whitfield (2007) discuss that nightjars avoid movement because they may in part rely on their cryptic plumage to avoid detection, therefore, records of AD may be unreliable for this species as passive disturbance is very hard to detect.
In a study investigating nightjar predation within forest habitats in England, Dolman (2010) recorded no evidence to show that recreational disturbance caused birds to flush close to paths or that nightjar breeding success was impacted by disturbance; the authors found that nightjar nests were only predated by mammalian predators (primarily fox and badger), with no predation by crow or any other diurnal avian predator and no instances of flushing by dogs were observed.
However, conversely, other studies have shown that nightjars are impacted by disturbance and breeding success is known to be lower in areas where there are high levels of human recreation. In a study investigating the effects of recreational disturbance on breeding nightjars on heathland sites in England, Langston et al. (2007) found that failed nests were significantly closer to paths than successful nests (median distance from nearest path = 45m for unsuccessful nests (n = 26) versus 150m for successful nests). Langston et al. (2007) also found that nightjar nests surrounded by a greater total path length were associated with higher losses (mainly due to predation by corvids); the authors suggested that paths should be buffered by 150m to protect breeding nightjars from dogs and pedestrians. In a similar study involving the same habitat in England, Murison (2002) also showed that sites with no public access had significantly higher breeding success than sites with open access; nightjar density was lower within 500m of heavily traversed pathways and nest failures were found up to 225m from paths. Along routes with known territories and nest sites adjacent to paths, Murison (2002) suggested that dogs should be kept on leads or excluded from key sites between May and August to protect breeding nightjars. In another study on English heathland habitat, Liley and Clarke (2003) found that nightjar density was lower within 500m of urban development, although this may have been at least partly due to a lack of woodland near urban developments which is one of the preferred foraging habitats of nightjars.
In a long-term study (10 years) at Sherwood Pines Forest Park in Nottinghamshire, Lowe and Durrant (2014) found that breeding nightjar density significantly decreased in areas that were heavily disturbed by recreational activities; the authors suggested that human recreational disturbance may drastically alter settlement patterns and the nest site selection of arriving females and that buffer zones around territories should be based on the response to disturbance of females rather than males.
Likely sensitivity to disturbance = Medium/High
Quantitative information = Medium agreement & Limited evidence
Breeding season buffer zone = 150-500m
Nightjar is assessed to have a medium to high sensitivity to human disturbance.
Quantitative studies measuring AD/FID are very limited for nightjar; a mean FID value recorded for nightjar is 10m when approached by a pedestrian during the breeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for nightjar during the breeding season is 150 to 300m although they noted that ‘estimates of static disturbance distances should be viewed with some scepticism because avoiding any movement is probably part of the suite of behaviours nightjars use to escape detection. This trait is also likely to lead to low active disturbance distances, with birds only flushing from the nest when an approaching potential predator is close’. Buffer zones for nightjar range from 150 to 500m for pedestrian disturbance and 50 to 200m for forestry operations.
In the UK, nightjar has the potential to be disturbed on breeding grounds. A buffer zone of 150-500m is suggested to protect breeding nightjar from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions.
Knowledge gaps
Further AD/FID studies required during the breeding season investigating a range of disturbance sources.
Kingfisher, Alcedo atthis
Conservation Status
UK: Green List, Schedule 1
European: Least Concern, Annex 1
UK status
Migrant/Resident Breeder
UK and Scottish population estimate
UK population = 3,850-6,400 breeding pairs (Woodward et al., 2020); Scottish population = 330-450 breeding pairs, 1,200-1,800 individuals in winter (Forrester et al., 2012).
UK long-term trend
Breeding range has fluctuated over the last 40 years, losses have generally outweighed gains although there have been gains in eastern areas of England and Scotland (Balmer et al., 2013). Breeding numbers increased between the mid-1980s and 2005, but since this time numbers have fallen (Balmer et al., 2013). Wintering distribution increased between 1981/84 and 2007/11, possibly linked to milder winters (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
Kingfisher was not included in Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in a rural habitat in Spain: FID = 24m (n = 1) (Díaz et al., 2021).
Surveyor walking in an urban habitat in France: Mean FID = 9.5m (n = 2), Min/Max FID = 5 to 14m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Poland: FID = 24.6m (n = 1) (Díaz et al., 2021).
Nonbreeding season:
Surveyor walking in Europe: FID = 24m (n = 1) (Møller and Erritzøe, 2010).
Surveyor walking in Europe: Mean FID = 16.27m (n = 2) (Møller, 2008a).
Surveyor walking in a range of habitats in Sir Lanka: Mean FID = 14.8 (n = 8); Min/Max FID = 3 to 26m (Gnanapragasam et al., 2021).
Nonbreeding season (Azure kingfisher, Ceyx azureus, stand in species for European kingfisher):
Surveyor walking in a range of habitats in Australia: Mean FID = 11.7m (n = 10) (Weston et al., 2012).
Unknown season (Malachite kingfisher, Alcedo cristata, stand in species for European kingfisher):
Surveyor walking in Africa: Mean FID = 10.3m (n = 4) (Weston et al., 2021).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone available for kingfisher.
Ecology and non-quantitative information on disturbance responses
Common kingfishers are resident birds in the UK which inhabit lowland river areas. This species is one of the most northerly members of a mainly tropical family, the ispida race is present in the UK and much of Europe, but is replaced in the Mediterranean Basin by the nominate atthis which also breeds in central Asia (Wernham et al., 2002). Kingfisher is absent from the Scottish Highlands and islands, but in lowland areas of England and Wales it is widespread; only a small population is present in Scotland which is concentrated on the mainland mainly in the southern and eastern lowlands (Balmer et al., 2013), but smaller numbers are also found north to the Moray Firth (Forrester et al., 2012). Preferred habitats of this species are still or gently flowing freshwater streams, small rivers, canals, drains and ditches where birds can plunge dive from a perch to catch small fish and aquatic insects, although occasionally insects may be caught in the air (Snow and Perrins, 1998). Kingfishers breed in tunnels that are excavated into steep or vertical banks, usually (but not always) over water (Snow and Perrins, 1998). In the UK, this species is mainly sedentary, although juveniles disperse away from breeding territories; some kingfishers move to coastal habitats in winter, although generally distribution is similar in both the breeding and nonbreeding seasons (Balmer et al., 2013). Migration is rare in the UK, although some individuals may cross the English Channel or the North Sea (Wernham et al., 2002).
Kingfishers are shy, reclusive birds and are potentially sensitive to human disturbance, particularly during the breeding season. If the presence of humans prevents kingfishers from entering their nests for extended periods of time, chicks may weaken from cold or hunger and reduce their begging calls, which in turn may stimulate the parents to provide less food (RSPB, 2021c). Kingfishers may not nest in areas if there is ongoing disturbance nearby; a study on watercourses in Ireland indicated that kingfisher numbers were lowest in areas that had the highest percentage of paths and tracks, roads and human trampling, which may suggest that such disturbances could be having a negative effect on the kingfisher population, although low fish densities also likely impacted kingfisher density in the Irish study (BirdWatch Ireland, 2010). A study in Spain indicated that the highest densities of kingfishers are located along rivers with the lowest human population density as well as minor agricultural use, indicating that this species prefers more pristine watercourses (Peris and Rodriguez, 1997). However, kingfishers can breed successfully on rivers within urban areas such as the River Kelvin in Glasgow and the Rivers Black Cart and White Cart in Paisley, and appear to be unaffected by people walking along the riverbank paths, possibly because the rivers are wide enough to mitigate disturbance.
A number of studies in Asia have investigated the impact of human disturbance on common kingfishers. In a study in Dhaka, Bangladesh, investigating daily activity patterns of common kingfishers, Sultana and Sarker, (2016) found that kingfishers were more active in the morning compared with the afternoon, which the authors suggested was due to increased human presence and high traffic noise along waterbodies during the afternoon. Biswas and Rahman (2012) estimated that approximately 15% of the major threats for kingfishers at Chittagong University in Bangladesh were due to human disturbance around nesting, feeding and roosting areas, as well as some public superstition and dislike towards kingfishers. Noor et al. (2014) found that kingfisher density was low in areas with high levels of vehicular traffic and human habitation along the bank of the Dal Lake in Jammu and Kashmir, India.
Likely sensitivity to disturbance = Low/Medium
Quantitative information = High agreement & Limited evidence
Breeding season buffer zone = 50-100m
Nonbreeding season buffer zone = 50-100m
Kingfisher is assessed to have a low to medium sensitivity to human disturbance.
The maximum FID value recorded for kingfisher when approached by a pedestrian is 25m during the breeding season and 26m during the nonbreeding season. There are no published buffer zones for kingfisher.
In the UK, kingfisher has the potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season; as a hole nesting species kingfisher may be less likely to be disturbed when on the nest. Depending on the level of habituation to disturbance, a minimum buffer zone of 50-100m is suggested to protect breeding kingfisher from pedestrian disturbance, but further studies on the impacts of human disturbance are required to help inform such decisions.
Knowledge gaps
Further AD/FID studies required during the breeding and nonbreeding seasons to investigate a range of disturbance sources.
Crested tit, Lophophanes cristatus
Conservation Status
UK: Green List, Schedule 1
European: Least Concern
UK status
Resident Breeder
UK and Scottish population estimate
UK population = 1,000-2,000 breeding pairs in Scotland (Woodward et al., 2020; Forrester et al., 2012); Scottish winter population = 5,600-7,900 individuals in winter (Forrester et al., 2012).
UK long-term trend
Crested tit was probably widespread in Scotland when ancient native pinewood covered much of the highlands, but this species declined and fragmented as the forest was cut down (Forrester et al., 2012). However, new pine plantations planted in the 20th century have allowed the range to extend again and it is likely that the population has also increased (Forrester et al., 2012). The Scottish breeding range increased by 28% between 1968/72 and 2007/11 and the wintering range expanded by 50% between 1981/84 and 2007/11 (Balmer et al., 2013).
AD/FID
Quantitative disturbance distances
FID updates (Jiang and Møller, 2017; Møller, 2008a; Dolman, 2010) published since Ruddock and Whitfield (2007).
Breeding season:
Surveyor walking in Europe: Mean FID = 6.2m (n = 34) (Jiang and Møller, 2017).
Pedestrian leisure (unspecified) in Denmark: Mean FID = 6.08m (n = 7) (Møller et al., 2007).
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD = 75m (n = 9); Min/Max AD (80% opinion range) = <10 to 100m; Min/Max AD (90% opinion range) = 50 to 100m.
Range of Median FID = 5 to 30m (n = 10); Min/Max FID (80% opinion range) = <10 to100m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Breeding season (Willow tit, Parus montanus, stand in species for crested tit):
Surveyor walking in Europe: Mean FID = 5.6m (n = 7) (Jiang and Møller, 2017).
Breeding season (Marsh tit, Parus palustris, stand in species for crested tit):
Surveyor walking in Europe: Mean FID = 6.3m (n = 40) (Jiang and Møller, 2017).
Breeding season (Blue tit, Parus caeruleus, stand in species for crested tit):
Surveyor walking in Europe: Mean FID = 5.4m (n = 262) (Jiang and Møller, 2017).
Breeding season (Coal tit, Periparus ater, stand in species for crested tit):
Surveyor walking in Europe: Mean FID = 5.8m (n = 13) (Jiang and Møller, 2017).
Breeding season (Great tit, Parus major, stand in species for crested tit):
Surveyor walking in Europe: Mean FID = 5.9m (n = 450) (Jiang and Møller, 2017).
Nonbreeding season:
Surveyor walking in Europe: Mean FID = 6.32m (n = 18) (Møller and Erritzøe, 2010).
Surveyor walking in Europe: Mean FID = 6.08m (n = 7) (Møller, 2008a).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone updates published since Ruddock and Whitfield (2007).
Breeding season:
Forestry operations in the UK: Safe working distance = 50 to 200m (Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Ecology and non-quantitative information on disturbance responses
In the UK, crested tit is a resident species confined to pinewoods of northern Scotland; the core range covers the Caledonian pinewoods of upper Strathspey and pinewoods of lower Strathspey; the scoticus race occurs almost exclusively in native pinewoods and Scots pine plantations in the coastal plains of Moray and Nairn (Balmer et al., 2013; Wernham et al., 2002). Smaller numbers of crested tits are also recorded in pine plantations in Easter Ross and east Inverness-shire, as well as remnant pine forests of the glens from Strathbran and Strathfarrar south to Glen Garry (Balmer et al., 2013). The density of wintering crested tits has been found to be ten times higher in ancient native pinewoods compared with planted pinewoods (Summers et al., 1999). Crested tit is a hole nesting species, generally in rotten tree stumps, and nest boxes are regularly used (Thom, 1986). Food is mainly insects and spiders, although plant material (mainly conifer seeds) may be eaten outside of the breeding season (Snow and Perrins, 1998), this species often forages on the ground or in low branches (Svensson et al., 2009). Adult crested tits are sedentary and although juveniles disperse over short distances post-breeding, breeding and nonbreeding distributions are similar (Balmer et al., 2013).
Crested tits can be tolerant of human presence; there are a number of records of birds visiting garden bird tables and feeders on Skye and in Gairloch, (Balmer et al., 2013) the RSPB Loch Garten Nature Centre in Speyside and in Moray (Forrester et al., 2012), particularly during the winter (Highland Nature, 2014) although Svensson et al. (2009) mentions that this behaviour is relatively rare. Like other species of the tit family, crested tits can be very inquisitive and at times may approach humans making a noise, but this behaviour depends on the stage of nesting; in the spring this species can be very elusive and difficult to find (Highland Nature, 2014). Svensson et al. (2009) note that crested tits are usually difficult to approach, although this species is not known to be particularly shy.
In studies using distance sampling analysis to estimate the density of crested tits in Scotland, the distance at which a pedestrian walking a transect line could detect a crested tit ranged between 39.3 to 62.5m; tits recorded along transects are usually detected by a contact or scolding call and therefore FID values are likely to be lower than detection distances (see summary in Ruddock and Whitfield, 2007; Calladine 2006; Summers et al., 1999).
Likely sensitivity to disturbance = Low
Quantitative information = High agreement & Limited evidence
Breeding season buffer zone = 10-50m
Nonbreeding season buffer zone = 10-50m
Crested tit is assessed to have a relatively low sensitivity to human disturbance.
Quantitative studies measuring AD/FID are limited for crested tit; the maximum mean FID value recorded for this species when approached by a pedestrian is 6.2m during the breeding season and 6.3m during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for crested tit during the breeding season is 50-100m. Buffer zones for crested tit range from 50 to 200m for forestry operations.
In the UK, crested tit may have some potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 10-50m is suggested to protect breeding and nonbreeding crested tits from pedestrian disturbance.
Knowledge gaps
Further AD/FID studies required during the breeding and nonbreeding seasons to investigate a range of disturbance sources.
Crossbill species, Loxia spp.
Conservation Status
Common crossbill (Loxia curvirostra):
UK: Green List, Schedule 1
European: Least Concern
Scottish crossbill (Loxia scotica):
UK: Amber List, Schedule 1
European: Least Concern, Annex 1
Parrot crossbill (Loxia pytyopsittacus):
UK: Amber List
European: Least Concern
UK status
Common crossbill:
Migrant/Resident Breeder, Passage/Winter Visitor
Scottish crossbill:
Endemic (Scotland) breeder
Parrot crossbill: Scarce Visitor, Occasional Breeder (Scotland)
UK and Scottish population estimate
Common crossbill:
Breeding: UK = 26000 pairs; Scotland = 5,000 to 50,000 pairs depending on cone crops elsewhere in Europe and in UK (Forrester et al., 2007).
Scottish crossbill:
Breeding Scotland only = 300 to 1,300 pairs (Forrester et al., 2007).
Parrot crossbill:
Breeding: Scotland only = ca. 100 pairs
UK long-term trend
Huge fluctuations, but also a long-term (20th and 21st century) increase in common crossbill numbers and range relating to increase in amount of mature plantation forestry (Balmer et al., 2013; Forrester et al., 2007).
AD/FID
Quantitative disturbance distances
FID update (Díaz et al., 2021; Møller and Erritzøe, 2010; Møller, 2008b; Møller et al., 2007) published since Ruddock and Whitfield (2007).
Breeding season (common crossbill, Loxia curvirostra):
Surveyor walking in a rural habitat in Denmark: Range of mean FID = 4.7 to 5.5m (n = 7); Min/Max FID = 4.1 to 8.2m (Díaz et al., 2021).
Surveyor walking in a rural habitat in Spain: Mean FID = 9.2 to 16.4 (n = 4); Min/Max FID = 6.4 to 16.4m (Díaz et al., 2021).
Pedestrian (general) in Denmark: Mean FID = 4.6m (n = 12) (Møller et al., 2007).
Breeding season (parrot crossbill, Loxia pytyopsittacus):
Surveyor walking in a rural habitat in Denmark: Mean FID = 4.2m (n = 2); Min/Max FID = 2.8 to 5.72m (Díaz et al., 2021).
Breeding season (crossbill spp, Loxia spp):
Pedestrian walking/running, disturbance estimated by expert opinion:
Median AD = 5m (n = 16); Min/Max AD (80% opinion range) = <10 to 150m; Min/Max AD (90% opinion range) = 100 to 150m.
Median FID = 5m (n = 17); Min/Max FID (80% opinion range) = <10 to 150m.
(Ruddock and Whitfield, 2007; Whitfield et al., 2008a).
Nonbreeding season (common crossbill, Loxia curvirostra):
Pedestrian (general) in Europe: Mean FID = 4.74m (n = 2) (Møller and Erritzøe, 2010).
Pedestrian (general activity) in Europe: Mean FID = 4.73m (n = 2) (Møller, 2008b).
MAD and/or
Buffer zone
Quantitative distances
No MAD or buffer zone updates published since Ruddock and Whitfield (2007).
Breeding season (common crossbill, Loxia curvirostra):
Forestry operations in Canada: Buffer zone = 70m (Waterhouse and Harestead, 1999).
Forestry operations in the UK: Safe working distance = 50 to 150m
(Currie and Elliot, 1997; Forestry Commission Scotland, 2006).
Breeding season (Scottish crossbill, Loxia scotica):
Forestry operations in the UK: Safe working distance = 150 to 300m
(Currie and Elliot, 1997).
Forestry operations in Scotland: Safe working distance = 50 to 150m
(Forestry Commission Scotland, 2006).
Breeding season (parrot crossbill, Loxia pytyopsittacus):
Forestry operations in Scotland: Safe working distance = 50 to 150m
(Forestry Commission Scotland, 2006).
Ecology and non-quantitative disturbance responses
Where ranges overlap, common and Scottish crossbills cannot reliably be told apart using visual identification, however, Scottish crossbills are limited in range to northeast Scotland and the eastern Highlands, so outside of this range, records refer solely to common crossbills (Balmer et al., 2013). Crossbills are associated with conifer plantations and are widely distributed throughout most of Scotland and Wales, exceptions are treeless areas of northwest Scotland, Northern Isles and some Hebridean islands (Balmer et al., 2013). Distribution in England is patchy, some of the higher densities are in conifer plantations in Norfolk, Hampshire and Dorset (Balmer et al., 2013). Crossbills forage by extracting seeds from conifers, this species may start breeding as early as midwinter, depending on availability of conifer seeds and consequently, breeding and nonbreeding distributions in the UK are fairly similar (Balmer et al., 2013; Snow and Perrins, 1998). Within northern Europe, this species feeds mainly on the seeds of Norway spruce, whereas the larger-billed parrot crossbill and Scottish crossbill are able to extract seeds from the tougher cones of Scots pine (Summers, 2018). Crossbills build nests high in conifer trees (Snow and Perrins, 1998).
Common crossbills can be found in deep dense forest, woodland edges or detached stands, they appear to tolerate human disturbance as they can be found in mature conifers in small towns and they will occasionally use overhead cables for perching or drinking from roof-top water tanks (Snow and Perrins, 1998). Crossbills are rarely found on the ground and disturbance studies on crossbill spp. indicate that human disturbance distances are relatively low (Díaz et al., 2021; Møller and Erritzøe 2010; Møller, 2008b; Møller et al., 2007), likely because their foraging and breeding habitat high up in trees keeps crossbills at a distance from human disturbance.
Likely sensitivity to disturbance = Low
Quantitative information = Medium agreement & Medium evidence
Breeding season buffer zone = 50-200m
Nonbreeding season buffer zone = 50-200m
Crossbill species are assessed to have a relatively low sensitivity to human disturbance.
The maximum FID value recorded for crossbill species when approached by a pedestrian is a maxmum of 16.4m during the breeding season and a mean of 4.7m during the nonbreeding season. Ruddock and Whitfield (2007) considered from expert opinion that the upper pedestrian disturbance distance limit for crossbill species during the breeding season is 100 to 150m, which is consistent with safe working distances used by Forestry Commission Scotland. Currie and Elliot (1997) suggest that safe working distances should be larger for Scottish crossbill (up to 300m), likely due to species differences in conservation status (Ruddock and Whitfield, 2007).
In the UK, crossbill species may have some potential to be disturbed on breeding grounds as well as on foraging and roosting grounds during the nonbreeding season. Depending on the level of habituation to disturbance, a buffer zone of 50-200m is suggested to protect breeding and nonbreeding crossbills from pedestrian disturbance.
Knowledge gaps
Lack of studies measuring AD/FID for Scottish crossbills during the breeding season.
Recommendations for further research
It has been acknowledged that all bird species assessed in this review are likely to vary their response to human disturbance in different areas due to differing levels of habituation between individuals as well as a wide range of other factors that can influence behavioural responses to disturbance (see ‘Habituation and other factors influencing disturbance distance’ section). Furthermore, this review has identified that there are a number of bird species where quantitative data on disturbance distances in relation to human activities are lacking (see ‘Data gaps’ section). Therefore, due to these variable factors and data gaps, the range of disturbance distances presented in this review are intended as a guide only. For studies that require to understand more precisely the distance a focal species will respond to a given source of disturbance under a given set of environmental conditions, specific bird disturbance distance studies need to be carried out on a site-specific basis.
Future disturbance distance studies investigating the impacts of human activity on bird disturbance should aim to record quantitative records of disturbance distances in terms of AD and FID. These measures of disturbance distances can be recorded by measuring the distance between a source of disturbance and the position of a focal bird when 1) the focal bird is first alerted to the source of disturbance (AD) and 2) when the focal bird first responds to the source of disturbance by moving away (FID). FID should still be recorded even if it is not possible to record AD; AD is usually more difficult to determine than FID, as alert behaviour is often cryptic compared with the FID response of physically moving away from the source of disturbance.
Standardised data should be collected in order to efficiently compare data recorded in different disturbance distance studies. Any study aiming to deliberately disturb birds in Scotland should also discuss the plan with NatureScot in advance in order to ensure that the work is compliant with legislation and with conservation objectives and welfare considerations. The following list provides a guide to basic information that should be recorded at the time of a disturbance distance study:
- Focal bird species, and age/sex of bird where that can be determined from plumage;
- Study location;
- Date;
- Weather conditions;
- Details of the source of disturbance (e.g. person walking, dog running, rock climber, motorboat, canoe, drone etc. moving towards focal bird);
- Whether the source of disturbance is visual or acoustic or both;
- AD distance (if it is possible to identify);
- FID distance; and
- Whether the study location is likely to be disturbed or undisturbed; if it is disturbed then what the likely source of disturbance is (e.g. is the study location frequented by people/boats/aircraft etc., or is it a remote and relatively undisturbed site).
Secondary factors that would be useful to record at the time of a disturbance distance study include the following:
- The initial distance between the source of disturbance and the focal bird (i.e. the study starting distance before the point of AD or FID has been reached);
- A record of whether the focal bird is likely to be breeding or nonbreeding;
- Specific habitat of the study location (e.g. sandy beach, cliffs, estuary mudflats etc.);
- Time of day;
- Tidal state (where coastal);
- Type of behaviour focal bird is displaying before the disturbance event (e.g. foraging/roosting/nesting/loafing);
- Type of AD behaviour (e.g. head-up, alarm calling, aggressive display, unknown);
- Type of FID behaviour (e.g. walk/run away, fly away 50m, swim/dive away from source of disturbance);
- Whether the focal bird is alone or with other birds (if it is the latter, then record the identity of other bird species and the flock size); and
- Length of time spent flying away from the source of disturbance.
Outside the field of applied impact assessments and academic research, there is also a need to record disturbance distances for bird species in a range of study locations under a variety of environmental conditions (including different seasons and weather conditions) in order to better understand the realistic range of natural disturbance distances. Disturbance distance studies do not necessarily involve sophisticated equipment or a particular knowledge of disturbance-based research. Disturbance distance studies can be carried out by anybody who can use a measuring device (e.g. a measuring tape or a range finder) and who has a good knowledge of bird species identification. Disturbance distance studies would, therefore, be highly appropriate as a Citizen Science project to build up a more detailed picture of sensitivity of birds to human disturbance. Alternatively, studies of disturbance responses would make excellent undergraduate or Masters research projects. Collating disturbance responses into one database will help to build a clearer picture of the potential impacts of disturbance on birds caused by human activities.
References
Ackerman, J.T., Takekawa, J.Y., Kruse, K.L., Orthmeyer, D.L., Yee, J.L., Ely, C.R., Ward, D.H., Bollinger, K.S. and Mulcahy, D.M. 2004. Using radiotelemetry to monitor cardiac response of free-living tule greater white-fronted geese (Anser albifrons elgasi) to human disturbance. Wilson Bulletin 116: 146–151.
Adams, J.K. and Scott, V.E. 1979. Timber harvest modification around an active osprey nest. Western Birds 10: 157–158.
Allport, G. 2016. Fleeing by whimbrel Numenius phaeopus in response to a recreational drone in Maputo Bay, Mozambique. Biodiversity Observations 7.44,1-5.
Althouse, M.A., Cohen, J.B., Karpanty, S.M., Spendelow, J.A., Davis, K.L., Parsons, K.C. and Luttazi, C.F. 2019. Evaluating response distances to develop buffer zones for staging terns. Journal of Wildlife Management 83:260–271.
Alves, M., Ferreira, J.P., Torres, I., Fonseca, C. and Matos, M. 2014. Habitat use and selection of the marsh harrier Circus aeruginosus in an agricultural-wetland mosaic. Ardeola 61: 351-366.
Anonymous. 2012. Maintaining the integrity of northern goshawk nesting and post-fledging areas in the ecosystem based management plan area of coastal British Columbia: guidance for forest professionals. Found, Used in BDR database.
Arlettaz, R., Patthey, P. and Braunisch, V. 2013. Impacts of outdoor winter recreation on alpine wildlife and mitigation approaches: a case study of the black grouse. In The impacts of skiing and related winter recreational activities on mountain environments. Ebooks. Pp 137–154.
Arnold, J.A., Nisbet, I.C.T. and Hatch, J.J. 1998. Are common terns really indeterminate layers? Responses to experimental egg removal. Colonial Waterbirds 21: 81-86.
Australian Government. 2021. Species profile and threats database - Wood sandpiper
Azaki, B.D.A. and Cresswell, W. 2021. Level of local human disturbance and feeding state determines escape behaviour in Eurasian oystercatchers. Ethology 127: 986-994.
Baines, D. and Richardson, M. 2007. An experimental assessment of the potential effects of human disturbance on black grouse Tetrao tetrix in the North Pennines, England. Ibis 149: 56–64.
Balmer, D.E., Gillings, S., Caffrey, B.J., Swann, R.L., Downie, I.S. and Fuller, R.J. 2013. Bird Atlas 2007-11: the breeding and wintering birds of Britain and Ireland. BTO Books, Thetford.
Baltic, M. 2005. Impact of human disturbance on Alpine wildlife in winter, stress, activity and energetics in the endangered black grouse Tetrao tetrix. PhD thesis, University of Bern.
Baltic, M., Jenni-Eiermann, S., Arlettaz, R. and Palme, R. 2005. A non-invasive technique to evaluate human-generated stress in the black grouse. Annals of New York Academy of Sciences 1046: 81-95.
Batten, L.A. 1977. Sailing on reservoirs and its effects on water birds. Biological Conservation 11: 49–58.
Baudains, T.P. and Lloyd, P. 2007. Habituation and habitat changes can moderate the impacts of human disturbance on shorebird breeding performance. Animal Conservation 10: 400-407.
Baxter, E.V. and Rintoul, L.J. 1953. The Birds of Scotland. Oliver and Boyd, Edinburgh.
BCM Environmental Services Limited. 2011. Report on the delivery of a monitoring programme for bean goose on the Slamannan Plateau 2010/2011. Scottish Natural Heritage Commissioned Report No. 487.
Beale, C.M. and Monaghan, P. 2004. Behavioural responses to human disturbance: a matter of choice? Animal Behaviour 68: 1065-1069.
Beaman, M. and Madge, S. 1998. The Handbook of Bird Identification for Europe and the Western Palearctic. Helm, London.
Becker, D.M. and Ball, I.J. 1983. Merlin (Falco columbarius). Impacts of coal surface mining on 25 migratory bird species of high federal interest, edited by Armbruster, J.S. USFWS pp 124-137.
Bell, D. V., and Austin, L. W. 1985. The game-fishing season and its effects on overwintering wildfowl. Biological Conservation 33:65·80.
BirdLife International. 2021a. European Red List of Birds. Luxembourg: Publications Office of the European Union.
BirdLife International. 2021b. Data Zone.
BirdWatch Ireland. 2010. Assessment of the distribution and abundance of Kingfisher Alcedo atthis and other riparian birds on six SAC river systems in Ireland. A report commissioned by the National Parks and Wildlife Service.
Biswas, J.K. and Rahman, M.M. 2012. Status, habitats and threats of kingfishers in Chittagong University campus. Bangladesh Journal of Environmental Science 23, 114-118.
Black, J.M. and Rees, E.C. 1984. The structure and behaviour of the Whooper Swan population wintering at Caerlaverock, Dumfries and Galloway, Scotland: an introductory study. Wildfowl 35, 21-36.
Blumstein, D.T. 2006. Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Animal Behaviour 71: 389-399.
Blumstein D.T. 2003. Flight-initiation distance in birds is dependent on intruder starting distance. Journal of Wildlife Management 67:852–857.
Blumstein, D.T., Fernández-Juricic, E., LeDee, O., Larsen, E., Rodriguez-Prieto, I. and Zugmeyer, C. 2004. Avian risk assessment: effects of perching height and detectability. Ethology 110: 273-285.
Blumstein D.T., Anthony L.L., Harcourt R. and Ross G. 2003. Testing a key assumption of wildlife buffer zones: is flight initiation distance a species-specific trait? Biological Conservation 110: 97–100.
Boeker, E.L. 1970. Use of aircraft to determine golden eagle, Aquila chrysaetos, nesting activity. Southwestern Naturalist 15: 136–137.
Boer, W. and Longamane, F. 1996. The exploitation of intertidal food resources in Inhaca Bay, Mozambique, by shorebirds and humans. Biological Conservation 78: 295–303.
Bogdanova, M.I., Newell, N., Harris, M.P., Wanless, S. and Daunt, F. 2014. Impact of visitor disturbance on Atlantic puffins and Arctic terns breeding on the Isle of May. Scottish Natural Heritage report.
Bogliani, G., Barbieri, F. and Tiso, E. 1994. Nest site selection by the hobby (Falco subbuteo) in poplar plantations in northern Italy. Journal of Raptor Research 28: 13-18.
Bolduc, F. and Guillemette, M. 2003. Human disturbance and nesting success of common eiders: interaction between visitors and gulls. Biological Conservation 110: 77–83.
Booms, T.L., Whitman, J.S. and Gardner, C.L. 2010. Utility of helicopters for short-eared owl nest searches and surveys. Journal of Raptor Research 44:247–248.
Borgmann, K.L. 2012. A Review of human disturbance impacts on waterbirds. Audubon Report.
Bötsch, Y., Tablado, Z., Scherl, D., Kéry, M., Graf, R.F and Jenni, L. 2018. Effect of recreational trails on forest birds: Human presence matters. Frontiers in Ecology and Evolution 175: 1-10.
Bradbury, G., Trinder, M., Furness, B., Banks, A.N., and Caldow, R.W.G. 2014. Mapping seabird sensitivity to offshore wind farms. PLoS ONE 9(9): e106366.
Brambilla, M., Rubolini, D. and Guidali, F. 2004. Rock climbing and raven Corvus corax occurrence depress breeding success of cliff-nesting peregrines Falco peregrinus. Ardeola 51: 425-430.
Brazil, M.A. 1981. The behavioural ecology of the whooper swan (Cygnus Cygnus). PhD thesis, University of Stirling.
Bregnballe, T., Aaen, K. and Fox, A.D. 2009. Escape distances from human pedestrians by staging waterbirds in a Danish wetland. Wildfowl (Special Issue 2): 115–130.
Brett, J. 2012. Disturbance to Wading birds Charadriiformes on their winter feeding grounds: Factors affecting behavioural response. A research paper as part of the requirement for the BSc (Hons) Ecology and Wildlife Conservation University of Bournemouth.
Briggs, B.D.J., Hill, D.A. and Gosler, A.G. 2012. Habitat selection and waterbody-complex use by wintering gadwall and shoveler in South West London: Implications for the designation and management of multi-site protected areas. Journal for Nature Conservation 20, 200–210.
Briggs, K. 1984. The breeding ecology of coastal and inland oystercatchers in north Lancashire. Bird Study 31: 141–147.
Bright, J.A., Langston, R.H.W. and Anthony, S. 2009. Mapped and written guidance in relation to birds and onshore wind energy development in England. RSPB Research Report No 35
Bright, J.A., Langston, R.H.W., Bullman, R., Evans, R.J., Gardner, S., Pearce-Higgins, J. and Wilson, E. 2006. Bird Sensitivity Map to provide locational guidance for onshore wind farms in Scotland. RSPB Research Report No 20.
British Columbia Ministry of Forests, Lands and Natural Resource Operations 2013. Guidelines for Raptor Conservation during Urban and Rural Land Development in British Columbia 2013.
Brown, A. and Grice, P. 2005. Birds in England. T&AD Poyser, London.
Bryant, D.M. and Leng, J. 1975. Feeding distribution and behaviour of shelduck in relation to food supply. Wildfowl, 26,20-30.
BTO. n.d. BirdFacts
Bundy, G. 1979. Breeding and feeding observations on the black-throated diver. Bird Study 26: 33-36.
Burger, J. and Gochfeld M. 1988. Defensive aggression in terns: effect of species, density and isolation. Aggressive Behavior 14: 169–178.
Burger, J. and Gochfeld, M. 1991. Reproductive vulnerability: parental attendance around hatching in roseate (Sterna dougallii) and common (S. hirundo) terns. Condor 93: 125-129.
Burger, J. 1998. Effects of motorboats and personal watercraft on flight behavior over a colony of common terns. Condor 100: 528–534.
Burton, N.H.K. 2006. The impact of the Cardiff Bay barrage on wintering waterbirds. In: Boere, G., Galbraith, C., Stroud, D. (ed.), Waterbirds around the world, pp. 805. The Stationary Office, Edinburgh, UK.
Burton, N. H. K., Armitage, M. J. S., Musgrove, A. J. and Rehfisch, M. M. 2002a. Impacts of man-made landscape features on numbers of estuarine waterbirds at low tide. Environmental Management 30: 857-864.
Burton, N.H.K. and Evans, P.R. 1997. Survival and winter site-fidelity of Turnstones Arenaria interpres and Purple Sandpipers Calidris maritima in northeast England. Bird Study 44, 35-44.
Burton, N.H.K., Evans, P.R. and Robinson, M.A. 1996. Effects on shorebird numbers of disturbance, the loss of a roost site and its replacement by an artificial island at Hartlepool, Cleveland. Biological Conservation 77: 193-201.
Burton, N.H.K., Rehfisch, M.M. and Clark, N.A. 2002b. Impacts of disturbance from construction work on the densities and feeding behavior of waterbirds using the intertidal mudflats of Cardiff Bay, U.K. Environmental Management 30: 865-871.
Byrkjedal, I. 1987. Antipredator behavior and breeding success in greater golden-plover and Eurasian dotterel. The Condor 89:40-47.
Calladine, J. 2006. Site condition monitoring of the breeding aggregation of crested tits (Parus cristatus) on the North Rothiemurchus Pinewood Site of Special Scientific Interest. Scottish Natural Heritage Commissioned Report No. 166.
Campbell, L.H. and Milne, H. 1977. Goldeneye feeding close to sewer outfalls in winter. Wildfowl 28: 81–85.
Carlen, E.J., Li, R. and Winchell, K.M. 2021. Urbanization predicts flight initiation distance in feral pigeons (Columba livia) across New York City. Animal Behaviour 178: 229-245.
Carless, S. 2005. The effect of human disturbance on the foraging behaviour of the oystercatcher Haematopus ostralegus on the rocky shore. A thesis submitted to the University of Plymouth.
Carney, K.M. and Sydeman, W.J. 1999. A review of human disturbance effects on nesting colonial waterbirds. Waterbirds 22: 68-79.
Carrier, W.D. and Melquist, W.E. 1976. The use of a rotor-winged aircraft in conducting nesting surveys of ospreys in northern Idaho. Raptor Research 10: 77–83.
Catt, D.C., Baines, D., Picozzi, N., Moss, R. and Summers, R.W. 1998. Abundance and distribution of capercaillie Tetrao urogallus in Scotland 1992–1994. Biological Conservation 85: 257–267.
Chabot, D., Craik, S.R. and Bird, D.M. 2015. Population census of a large common tern colony with a small unmanned aircraft. - PLoS One 10: 1–14.
Challis, A., Wilson, M.W., Schönberg, N., Eaton, M.A., Stevenson, A. and Stirling-Aird, P. 2020. Scottish Raptor Monitoring Scheme Report 2019. BTO Scotland, Stirling.
Charutha, K., Roshnath, R. and Sinu, P.A. 2021. Urban heronry birds tolerate human presence more than its conspecific rural birds. Journal of Natural History 55: 561-570.
Cheshire and Wirral Ornithological Society (CAWOS). 2019. Birds in Cheshire and Wirral - A breeding and wintering atlas.
Cocker, M. and Mabey, R. 2005. Birds Britannica. Chatto and Windus, London.
Coleman, R.A., Salmon, N.A. and Hawkins, S.J. 2003. Sub-dispersive human disturbance of foraging oystercatchers Haematopus ostralegus. Ardea 91: 263-268.
Collop, C., Stillman, R.A., Garbutt, A., Yates, M.G., Rispin, E. and Yates, T. 2016. Variability in the area, energy and time costs of wintering waders responding to disturbance. Ibis 158: 711-725.
Collop, C.H. 2016. Impact of human disturbance on coastal birds: population consequences derived from behavioural responses. PhD thesis, Bournemouth University.
Colwell, M. 2021. Beak tooth and claw: Living with predators in Britain. William Collins, London.
Congreve, W.M. and Freme, S.W.P. 1930. Seven weeks in eastern and northern Iceland. Ibis 6, 193-228.
Conomy, J.T., Collazo, J.A., Dubovsky, J.A. and Fleming, W.J. 1998. Dabbling duck behavior and aircraft activity in coastal North Carolina. The Journal of Wildlife Management 62, 1127-1134.
Conway, G.J., Burton, N.H.K., Handschuh, M. and Austin, G.E. 2008. UK population estimates from the 2007 breeding little ringed plover and ringed plover surveys. BTO Research Report No. 510.
Coppes, J., Ehrlacher, J., Thiel, D., Suchant, R. and Braunisch, V. 2017. Outdoor recreation causes effective habitat reduction in capercaillie Tetrao urogallus: a major threat for geographically restricted populations. Journal of Avian Biology 48: 1583–1594.
Craig, G.R. 2002. Recommended Buffer Zones and Seasonal Restrictions for Colorado Raptors. Colorado Division of Wildlife.
Cramp, S. and Simmons, K.E.L. (Eds.) 1985. The Birds of the Western Palearctic, Volume 4. Oxford University Press, Oxford.
Cramp, S. and Simmons, K.E.L. (Eds.) 1982. The Birds of the Western Palearctic, Volume 3. Oxford University Press, Oxford.
Cramp, S. and Simmons, K.E.L. (Eds.) 1977. The birds of the Western Palearctic, Volume 1. Oxford University Press, Oxford.
Cryer, M., Linley, N.W., Ward, R.M., Stratford, J., and Randerson, P.F. 1987. Disturbance of overwintering wildfowl by anglers at two reservoir sites in South Wales. Bird Study 34, 191-199.
Currie, F. and Elliot, G. 1997. Forests and birds - A guide for managing forests for rare birds.
D’Acunto, L.E., Spaul, R.J., Heath, J.A. and Zollner, P.A. 2018. Simulating the success of trail closure strategies on reducing human disturbance to nesting golden eagles. Condor 120: 703-718.
Dahl, E.L., Bevanger, K., Nygard, T., Roskaft, E. and Stokkem, B.G. 2012. Reduced breeding success in white-tailed sea eagles at Smöla windfarm, western Norway, is caused by mortality and displacement. Biological Conservation 145: 79-85.
Davidson, N.C. and Rothwell, P.I. 1993. Human disturbance to waterfowl on estuaries: conservation and coastal management implications of current knowledge. Wader Study Group Bulletin 68: 97-106.
de Jong, S., van den Burg, A. and Liosi, A. 2018. Determinants of traffic mortality of barn owls (Tyto alba) in Friesland, the Netherlands. Avian Conservation and Ecology 13: 2.
Dehnhard, N., Skei, J., Christensen‑Dalsgaard, S., May, R., Halley, D., Ringsby, T.H., Lorentsen, S-H. 2020. Boat disturbance effects on moulting common eiders Somateria mollissima. Marine Biology 167:12
Dennis, R.H. and Dow, H. 1984. The establishment of a population of goldeneyes Bucephala clangula breeding in Scotland. Bird Study 31: 217-222.
Díaz, M., Grim, T., Markó, G., Morelli, F., Ibáñez-Alamo, J.D., Jokimäki, J., Kaisanlahti-Jokimäki, M.L., Tätte, K., Tryjanowski, P. and Møller, A.P. 2021. Effects of climate variation on bird escape distances modulate community responses to global change. Scientific Reports 11: 12826.
Dierschke, V. 1994. Food and feeding ecology of purple sandpipers Calidris maritima on rocky intertidal habitats (Helgoland, German Bight). Netherlands Journal of Sea Research 31, 309-317.
Dierschke, V., Furness, R.W. and Garthe, S. 2016. Seabirds and offshore wind farms in European waters: Avoidance and attraction. Biological Conservation 202: 59-68.
Dolman, P. 2010. Woodlark and Nightjar recreational disturbance and nest predator study 2008 and 2009 Final Report to Breckland District Council.
Douglas, D.J.T., Bellamy, P.E. and Pearce-Higgins, J.W. 2011. Changes in the abundance and distribution of upland breeding birds at an operational wind farm. Bird Study 58: 37-43.
Duncan, M. and Marquiss, M. 1993. The sex/age ratio, diving behaviour and habitat use of Goldeneye Bucephala clangula wintering in northeast Scotland. Wildfowl 44: 111-120.
Dwyer, R.G. 2010. Ecological and anthropogenic constraints on waterbirds of the Forth Estuary: population and behavioural responses to disturbance. PhD Thesis, University of Exeter.
Eaton, M. and the Rare Breeding Birds Panel 2021. Rare breeding birds in the UK in 2019. British Birds 114: 646-704.
Eason, P., Rabea, B. and Attum, O. 2010.Conservation implications of flight initiation distance and refuge use in Corn Crakes Crex crex at a migration stopover site (Aves: Rallidae). Zoology in the Middle East 51, 9-14.
Ellenberg, U., Mattern, T. and Seddon, P.J. 2009. Habituation potential of yellow-eyed penguins depends on sex, character and previous experience with humans. Animal Behaviour 77: 289-296.
Ellis, D. H. 1982. The peregrine falcon in Arizona, habitat utilization and management recommendations. Institute for Raptor Studies, Research Report No 1.
Engelmoer, M. 2008. Breeding origins of wader populations utilizing the Dutch Wadden Sea, as deduced from body dimensions, body mass, and primary moult. PhD Thesis, University of Groningen.
Erwin, R. M. 1980. Breeding habitat use by colonially nesting waterbirds in two mid-Atlantic U.S. regions under different regimes of human disturbance. Biological Conservation 18: 39-51.
Erwin, R. M. 1989. Responses to human intruders by birds nesting in colonies: experimental results and management guidelines. Colonial Waterbirds 12:104–108.
European Commission, 2009. European Union Management plan 2009-2011. Technical Report – 2009-036.
European Commission, 2007a. Management plan for pintail (Anas acuta) 2007-2009. Technical Report - 004 – 2007.
European Commission, 2007b. Management plan for black-tailed godwit (Limosa limosa) 2007-2009. Technical Report - 019 – 2007.
European Commission, 2007c. Management plan for curlew (Numenius arquata) 2007-2009. Technical Report - 003 – 2007.
European Commission, 2010. Directive 2009/147/EC on the Conservation of Wild Birds.
Evans, D.M. and Day, K.R. 2002. Hunting disturbance on a large shallow lake: the effectiveness of waterfowl refuges. Ibis, 144, 2–8.
Everett, M.J. 1971. Breeding status of red-necked phalaropes in Britain and Ireland. British Birds 64, 293-302.
Ewins, P.J. 1997. Osprey (Pandion haliaetus) populations in forested areas of North America: Changes, their causes and management recommendations. Journal of Raptor Research 31:138-150.
Fearnley, H., Cruickshanks, K., Lake, S. and Liley, D. 2013. The effect of bait harvesting on bird distribution and foraging behaviour in Poole Harbour SPA. Unpublished report by Footprint Ecology for Natural England.
Feige, N., van der Jeugd, H.P., Voslamber, B. and Stahl, J. 2008. Characterisation of greylag goose Anser anser breeding areas in the Netherlands with special regard to human land use. Vogelwelt 129: 348–359.
Fernández, C. and Azkona, P. 1993. Human disturbance affects parental care of marsh harriers and nutritional status of nestlings. Journal of Wildlife Management 57: 602-608.
Fernandez-Bellon, D., Lusby, J., Bos, J., Schaub, T., McCarthy, A., Caravaggi, A., Irwin, S. and O’Halloran, J. 2021. Expert knowledge assessment of threats and conservation strategies for breeding hen harrier and short-eared owl across Europe. Bird Conservation International 31: 268-285.
Ferns, P.N. 2003. Plumage colour and pattern in waders. Wader Study Group Bulletin 100: 122-129.
Fielding, A. and Haworth, P. 2010. Farr windfarm: A review of displacement disturbance on golden plover arising from operational turbines between 2005-2009. Haworth Conservation.
Finney, S.K., Pearce-Higgins, J.W. and Yalden, D.W. 2005. The effect of recreational disturbance on an upland breeding bird, the golden plover Pluvialis apricaria. Biological Conservation 121: 53-63.
Fitzpatrick, S. and Bouchez, B. 1998. Effects of recreational disturbance on the foraging behaviour of waders on a rocky beach. Bird Study 45: 157-171.
Fleischner, T.L. and Weisberg, S. 1986. Effects of jet aircraft activity on bald eagles in the vicinity of Bellingham International Airport. Unpublished Report, DEVCO Aviation Consultants, Bellingham, WA. 12 pp.
Fletcher, R. J., McKinney, S. T. and Bock, C. E. 1999. Effects of recreational trails on wintering diurnal raptors along riparian corridors in a Colorado grassland. Journal of Raptor Research 33: 233-239.
Forestry Commission Scotland. 2006. Forest operations and birds in Scottish forests - the law and good practice.
Forgues, K. 2010. The effects of off-road vehicles on migrating shorebirds at a barrier island in Maryland and Virginia. Master thesis, Trent University, Peterborough, Ontario, Canada.
Forrester, R.W., Andrews, I.J., and McInerny, C.J. eds. 2007. The Birds of Scotland. The Scottish Ornithologists’ Club, Aberlady.
Forrester, R.W., Andrews, I.J., McInerny, C.J., Murray, R.D., McGowan, R.Y., Zonfrillo, B., Betts, M.W., Jardine, D.C. and Grundy, D.S. eds. 2012. The digital birds of Scotland. The Scottish Ornithologists’ Club, Aberlady.
Fox, A.D., Bell, D.V. and Mudge, G.P. 1993. A preliminary study of the effects of disturbance on feeding wigeon grazing on eel-grass Zostera. Wader Study Group Bulletin 68: 67-71.
Fox, A.D., Boyd, H., Walsh, A.J., Stroud, D.A., Nyeland, J. and Cromie, R.L. 2012. Earlier spring staging in Iceland amongst Greenland white-fronted geese Anser albifrons flavirostris achieved without cost to refuelling rates. Hydrobiologia 697: 103-110.
Fox, A.D. and Madsen, J. 1997. Behavioural and distributional effects of hunting disturbance on waterbirds in Europe: Implications for refuge design. Journal of Applied Ecology 34: 1-13.
Fox, A.D. and Stroud, D.A. 2002. Anser albifrons flavirostris Greenland white-fronted goose. BWP Update 4: 65-88.
Fraser, J.D., Frenzel, L.D. and Mathisen, J.E. 1985. The impact of human activities on breeding bald eagles in north-central Minnesota. Journal of Wildlife Management 49: 585-592.
Frikke, J. 1991. Breeding waders and wet grassland habitats in Denmark. Wader Study Group Bulletin 61, 42-49.
Frohlich, A. and Ciach, M. 2018. Noise pollution and decreased size of wooded areas reduces the probability of occurrence of tawny owl Strix aluco. Ibis 160: 634-646.
Fuller, R. J., Baker, J. K., Morgan, A. R., Scroggs, R. and Wright, M. 1985. Breeding populations of the hobby Falco subbuteo on farmland in the southern Midlands of England. Ibis 127: 510-516.
Furness, R.W. 1973. Roost selection by waders. Scottish Birds 7: 281-287.
Furness, R.W. 2016. Key pressures and threats faced by marine birds in the UK, conservation action for these birds, and identification of pressures and threats not effectively addressed by existing conservation action. Report to JNCC.
Furness, R.W. and Wade, H.M. 2012. Vulnerability of Scottish seabirds to offshore wind turbines. Report to Marine Scotland.
Furness, R.W., Wade, H.M. and Masden, E.A. 2013. Assessing vulnerability of marine bird populations to offshore wind farms. Journal of Environmental Management 119: 56-66.
Fyfe, R.W. and Olendorff, R.R. 1976. Minimizing the dangers of nesting studies to raptors and other sensitive species. Canadian Wildlife Service Occasional Paper No 23.
Galbraith, H., Hatch, J. J., Nisbet, I. C. T. and Kunz, T. H. 1999. Age-related changes in efficiency among breeding common terns Sterna hirundo: measurement of energy expenditure using doubly-labelled water. Journal of Avian Biology 30: 85.
Galeotti, P., Tavecchia, G. and Bonetti, A. 2000. Parental defence in long-eared owls Asio otus: effects of breeding stage, parent sex and persecution. Journal of Avian Biology 31: 431-440.
García, J., Miguélez, D., Astiárraga, H. and Zumalacárregui, C. 2015. Factors determining the occupation of marsh harrier Circus aeruginosus breeding grounds in a Mediterranean environment. Bird Study 62: 331–338.
Garthe, S., and Flore, B.O. 2007. Population trend over 100 years and conservation needs of breeding Sandwich terns (Sterna sandvicensis) on the German North Sea coast. Journal of Ornithology 148: 215-227.
Garthe, S., and Hüppop, O. 2004. Scaling possible adverse effects of marine wind farms on seabirds: developing and applying a vulnerability index. Journal of Applied Ecology 41: 724-734.
Gilbert, G., Gibbons, D.W. and Evans, J. 1998. Bird Monitoring Methods. RSPB.
Gill, J.A., Norris, K. and Sutherland, W.J. 2001. The effects of disturbance on habitat use by black-tailed godwits Limosa limosa. Journal of Applied Ecology 38: 846-856.
Gill, J.A., Sutherland, W.J. and Watkinson, A.R. 1996. A method to quantify the effects of human disturbance on animal populations. Journal of Applied Ecology 33: 786-792.
Gillings, S. and Fuller, R.J. 1999. Winter ecology of golden plovers and lapwings: A review and consideration of extensive survey methods. BTO Research Report No. 224.
Gittings, T., Peppiatt, C. and Troake, P. 2015. Disturbance response of great northern divers Gavia immer to boat traffic in inner Galway Bay. Irish Birds 10: 163-166.
Glover, H.K., Weston, M.A., Maguire, G.S., Miller, K.K. and Christie, B.A. 2011. Towards ecologically meaningful and socially acceptable buffers: Response distances of shorebirds in Victoria, Australia, to human disturbance. Landscape and Urban Planning 103: 326–334.
Gnanapragasam, J.J., Ekanyake, K.B., Ranawana, K., Symonds, M.R.E. and Weston, M.A. 2021. Civil war is associated with longer escape distances among Sri Lankan birds. American Naturalist 198: 653-659.
Goodship, N. and Furness, R.W. 2019. Seaweed hand-harvesting: literature review of disturbance distances and vulnerabilities of marine and coastal birds. Scottish Natural Heritage Research Report No. 1096.
Goss-Custard, J., Triplet, B., Sueur, F. and West, A. 2006. Critical thresholds of disturbance by people and raptors in foraging wading birds. Biological Conservation 127: 88–97.
Goss-Custard, J.D. 1981. Oystercatcher counts at roosts and at feeding grounds. British Birds 74: 197-199.
Götmark, F., Neergaard, R. and Ahlund, M. 1989. Nesting ecology and management of the Arctic loon in Sweden. Journal of Wildlife Management 53: 1025–1031.
Gregersen, J. 2006. The breeding population of Sandwich tern in Denmark, 1993-2005. Dansk Ornitologisk Forenings Tidsskrift 100: 88-96.
Griffin, L.R., Burrell, E.M., Harrison, A.L., Mitchell, C. and Hilton, G.M. 2020. Conservation management of Greenland white-fronted geese Anser albifrons flavirostris on Islay, Scotland. Scottish Natural Heritage Research Report No. 912.
Grubb, T. G., Delaney, D.K., Bowerman, W.W. and Wierda, M.R. 2010. Golden eagle indifference to heli-skiing and military helicopters in Northern Utah. Journal of Wildlife Management 74: 1275–1285.
Grubb, T.G. and King, R.M. 1991. Assessing human disturbance of breeding bald eagles with classification tree models. Journal of Wildlife Management 55: 500-511.
Grubb, T. G., Pater, L.L., Gatto, A.E. and Delaney, D.K. 2013. Response of nesting northern goshawks to logging truck noise in northern Arizona. Journal of Wildlife Management 77: 1618–1625.
Hancock, M. 2000. Artificial floating islands for nesting black-throated divers Gavia arctica in Scotland: construction, use and effect on breeding success. Bird Study 47: 165-175.
Haney, D.L. and White, C.M. 1999. Habitat use and subspecific status of merlins, Falco columbarius, wintering in central Utah. Great Basin Naturalist 59: 266-271.
Hardey, J., Crick, H., Wernham, C., Riley, H., Etheridge, B. and Thompson, D. 2013. Raptors: a field guide to survey and monitoring (3rd Edition). The Stationery Office, Edinburgh.
Harris, S.J., Massimino, D., Balmer, D.E., Eaton, M.A., Noble, D.G., Pearce-Higgins, J.W., Woodcock, P. & Gillings, S. 2020. The Breeding Bird Survey 2019. BTO Research Report 726. British Trust for Ornithology, Thetford.
Havera, S.P., Boens, L.R., Georgi, M.M. and Shealy, R.T. 1992. Human disturbance of waterfowl on Cacique Pool, Mississippi River. Wildlife Society Bulletin 20: 290-298.
Hearn, R.D. and Mitchell, C.R. 2004. Greylag goose Anser anser (Iceland population) in Britain and Ireland 1960/61 – 1999/2000. Waterbird Review Series, The Wildfowl and Wetlands Trust/Joint Nature Conservation Committee, Slimbridge.
Heggøy, O., and Øien, I. J. 2014. Conservation status of birds of prey and owls in Norway. NOF/BirdLife Norway-Report 1: 1–129.
Heimberger, M. Euler, D. and Barr, J. 1983. The impact of cottage development on common loon (Gavia immer) reproductive success in central Ontario, Canada. Wilson Bulletin 95: 431-439.
Herrmann, C., Nehls, H.W., Gregersen, J, Knief, W., Larsson, R., Elts, J., & Wieloch, M. 2008. Distribution and Population Trends of the Sandwich Tern Sterna sandvicensis in the Baltic Sea Area. Die Vogelwelt 129, 35-46.
Heppleston, P.B. 1972. The comparative breeding ecology of oystercatchers (Haematopus ostralegus) in inland and coastal habitats. Journal of Animal Ecology 41.
Highland Nature. 2014. Focus on crested tit
Hildén, O. and Vuolanto, S. 1972. Breeding biology of the red-necked phalarope Phalaropus lobatus in Finland. Ornis Fennica 49, 57-85.
Hirons, G., and Thomas, G. 1993. Disturbance on estuaries: RSPB nature reserve experience. Wader Study Group Bull. 68:72-78.
Hissa, R., Rintamaki, H., Virtanen, P., Linden, H. and Vihko, V. 2003. Energy reserves of the Capercaillie, Tetrao urogallus in Finland. Comparative Biochemistry and Physiology Part A: Physiology 97: 245-351.
Holloway, S. 1997. Winter Distribution and Disturbance of Wildfowl and Waders on Findhorn Bay. BTO Research Report No. 179.
Holm, T.E. and Laursen, K. 2009. Experimental disturbance by walkers affects behaviour and territory density of nesting Black-tailed Godwit Limosa limosa. Ibis, 151, 77–87.
Holmes, T., Knight, R.L. and Craig, G.R. 1993. Responses of wintering grassland raptors to human disturbance. Wildlife Society Bulletin 21: 461-468.
Horváth, Z., 2009. White-tailed eagle (Haliaeetus albicilla) populations in Hungary between 1987–2007. Denisia 27: 85–95.
Hume, R.A. 1976. Reactions of goldeneyes to boating. British Birds 69:178–179.
Jackson, D. 2018. Scapa Flow proposed Special Protection Area (pSPA): Inshore wintering waterfowl survey 2017/18. Scottish Natural Heritage Commissioned Report No. 1075.
Jackson, D. B. 2001. Experimental removal of introduced hedgehogs improves wader nest success in the Western Isles, Scotland. Journal of Applied Ecology 38: 802-812.
Jarrett, D., Cook, A.S.C.P., Woodward, I., Ross, K., Horswill, C., Dadam, D. and Humphreys, E.M. 2018. Short-term behavioural responses of wintering waterbirds to marine activity. Scottish Marine and Freshwater Science 9 (7).
Jiang, Y. and Møller, A.P. 2017. Antipredator escape distances of common and threatened birds. Behavioral Ecology 28: 1498–1503.
Johnston-Gonzalez, R. and Abril, E. 2019. Predation risk and resource availability explain roost locations of Whimbrel Numenius phaeopus in a tropical mangrove delta. Ibis 161, 839-853.
Joint Nature Conservation Committee (JNCC). 2012. Species Accounts.
Jørgensen, P.S., Kristensen, M.W. and Egevang, C. 2007. Dansk Ornitologisk Forenings Tidsskrift 101, 73-78.
Kaiser, M.J., Galanidi, M., Showler, D.A., Elliott, A.J., Caldow, R.W.G., Rees, E.I.S., Stillman, R.A. and Sutherland, W.J. 2006. Distribution and behaviour of common scoter Melanitta nigra relative to prey resources and environmental parameters. Ibis 148: 110–128.
Kalejta-Summers, B. and Chisholm, K. 2009. Numbers and distribution of breeding wood Sandpipers in Scotland - results of the 2007 national survey. Scottish Birds 29, 202-209.
Katzenberger, J.K. 2021. Habitat use and population viability of the red kite (Milvus milvus) in Germany. PhD thesis, University of Göttingen.
Keller, V. 1989. Variations in the response of great crested grebes Podiceps cristatus to human disturbance – a sign of adaptation. Biological Conservation 49: 31-45.
Keller, V. 1991. The effects of disturbance from roads on the distribution of feeding sites by geese (Anser brachyrhynchus, A. anser) wintering in northeast Scotland. Ardea 79: 229-232.
Kelly, L.M. 1992. The effects of human disturbance on common loon productivity in north western Montana. MSc thesis, Montana State University.
Kirby, J.S., Clee, C. and Seager, V. 1993. Impact and extent of recreational disturbance to wader roosts on the Dee Estuary: Some preliminary results. Wader Study Group Bulletin 68: 53-58.
Knapton, R.W., Petrie, S.A. and Herring, G. 2000. Human disturbance of diving ducks on Long Point Bay, Lake Erie. Wildlife Society Bulletin 28: 923-930.
Koch, S.L. and Paton, P.W.C. 2014. Assessing anthropogenic disturbances to develop buffer zones for shorebirds using a stopover site. Journal of Wildlife Management 78: 58-67.
Koivusaari, J., Nuuja, I. and Palokangas, R. 1988a. White-tailed eagle nesting sites in jeopardy. Suomen Luonto 47: 13-17.
Koivusaari, J., Nuuja, I. and Palokangas, R. 1988b. Three decades with the Sea eagles in Quark. Finlands Natur, 47: 4-7.
Konrad, P. M. 2004. Effects of management practices on grassland birds: Merlin. Northern Prairie Wildlife Research Center, Jamestown, ND. 20 pages.
Korschgen, C.E. and Dahlgren, R.B. 1992. Human Disturbances of Waterfowl: Causes, Effects, and Management. Waterfowl Management Handbook. 12.
Kortland, K. 2006. Forest management for capercaillie - An illustrated guide for forest managers.
Kortland, K., Evans, R., Douse, A. and Patterson, G. 2011. Managing forests for white-tailed eagles Practice note.
Langston, R.H.W., Liley, D., Murison, G., Woodfield, E. and Clarke, R.T. 2007. What effects do walkers and dogs have on the distribution and productivity of breeding European Nightjar Caprimulgus europaeus? - Ibis 149, 27–36.
Laiolo, P. and Rolando, A. 2005. Forest bird diversiy and ski-runs: a case of negative edge effect. Animal Conservation 7: 9-16.
Larsen, J.K. and Laubek, B. 2005. Disturbance effects of high-speed ferries on wintering sea ducks. Wildfowl 55: 99-116.
Laursen, K., Bregnballe, T., Therkildsen, O.R., Holm, T.E. and Nielsen, R.D. 2017. Disturbance of waterbirds by water-based recreational activities – a review (in Danish with English summary). Dansk Ornitologisk Forenings Tidsskrift 111: 96-112.
Laursen, K., Kahlert, J. and Frikke, J. 2005. Factors affecting escape distances of staging waterbirds. Wildlife Biology 11: 13-19.
Lethlean, H., van Dongen, W.F.D., Kostoglou, K., Guay, P-J. and Weston, M.A. 2017. Joggers cause greater avian disturbance than walkers. Landscape and Urban Planning 159: 42-47.
Levenson, H. and Koplin, J.R. 1984. Effects of human activity on productivity of nesting ospreys. Journal Wildlife Management 48: 1374-1377.
Liley, D. and Clarke, R.T. 2003. The impact of urban development and human disturbance on the numbers of nightjar Caprimulgus europaeaus on heathlands in Dorset, England. Biological Conservation, 114, 219-230.
Liley, D., Cruickshanks, K., Waldon, J. and Fearnley, H. 2011. Exe Estuary. Disturbance Study. Footprint Ecology Ltd., Wareham.
Liley, D. and Fearnley, H. 2012. Poole Harbour Disturbance Study. Report to Natural England. Footprint Ecology Ltd., Wareham.
Liley, D., Stillman, R.A. and Fearnley, H. 2010. The Solent Disturbance and Mitigation Project Phase II. Results of bird disturbance fieldwork, 2009/10. Footprint Ecology / Solent Forum.
Liley, D. and Sutherland, W.J. 2007. Predicting the population consequences of human disturbance for ringed plovers Charadrius hiaticula: a game theory approach. Ibis 149 (Suppl. 1): 82-94.
Lilleyman, A., Franklin, D.C., Szabo, J.K. and Lawes, M.J. 2016. Behavioural responses of migratory shorebirds to disturbance at a high-tide roost. Emu 116: 111-118.
Linssen, H., van de Pol, M., Allen, A.M., Jans, M., Ens, B.J., Krijgsvel, K.L., Frauendorf, M. and van der Kolk, H-J. 2019. Disturbance increases high tide travel distance of a roosting shorebird but only marginally affects daily energy expenditure. Avian Research 10: 31.
Liu, Y., Lu, Y., Chen, C., Zhang, G., Wang, Q., Xu, Z. and Wang, Z. 2018. Behavioural responses of the whooper swans Cygnus cygnus to human disturbance and their adaptability to the different habitats in the Rongcheng lagoon of China. Ecohydrology 11.
Livezey, K. B., Fernández-Juricic, E. and Blumstein, D.T. 2016. Database and metadata of bird flight initiation distances worldwide to assist in estimating human disturbance effects and delineating buffer areas. Journal of Fisheries and Wildlife Management 082015–JFWM–078.
Lowe, A. and Durrant, K. 2014. Effect of human disturbance on long-term habitat use and breeding success of the European Nightjar, Caprimulgus europaeus. Avian Conservation Ecology 9: 6.
Ma, M. and Cai, D. 2002. Threats to Whooper Swans in Xinjiang, China. Waterbirds: The International Journal of Waterbird Biology 25, 331-333.
MacLennan, A.M. and Evans, R.J. 2003. Public viewing of white-tailed sea eagles- take the birds to the people or the people to the birds? Sea Eagle 2000. Proceedings from the International Sea Eagle Conference, pp 417-422.
Madders, M. and Whitfield, D.P. 2006. Upland raptors and the assessment of wind farm impacts. Ibis 148: 43–56.
Madsen, J. 1985. Impact of disturbance on field utilization of pink-footed geese in West Jutland, Denmark. Biological Conservation 33: 53-63.
Madsen, J. 1994. Impacts of disturbance on migratory waterfowl. Ibis 137:567-574.
Madsen, J. 1998a. Experimental refuges for migratory waterfowl in Danish wetlands. I. Baseline assessment of the disturbance effects of recreational activities. Journal of Applied Ecology 35: 386-397.
Madsen, J. 1998b. Experimental refuges for migratory waterfowl in Danish wetlands. II. Tests of hunting disturbance effects. Journal of Applied Ecology 35: 398-417.
Madsen, J. and Fox, A. D. 1995. Impacts of hunting disturbance on waterbirds - a review. Wildlife Biology 1: 193-207.
Madsen, J., Tombre, I. and Eide, N.E. 2009. Effects of disturbance on geese in Svalbard: implications for regulating increasing tourism. Polar Research 28: 376–389.
Mak, B., Francis, R.A. and Chadwick, M.A. 2021. Living in the concrete jungle: a review and socio-ecological perspective of urban raptor habitat quality in Europe. Urban Ecosystems doi.org/10.1007/s11252-021-01106-6
Makarova, T. and Sharikov, A. 2015. Winter roost place selection of long-eared owls in European Russia. Journal of Raptor Research 49: 333–336.
Mallory, M.L. 2016. Reactions of ground-nesting marine birds to human disturbance in the Canadian Arctic. Arctic Science 2: 67-77.
Mallory, M.L., McNicol, D.K., Walton, R.A. and Wayland, M. 1998. Risk-taking by incubating common goldeneyes and hooded mergansers. Condor 100: 694-701.
Mallory, M.L. and Weatherhead, P.J. 1993. Observer effects on common goldeneye nest defence. Condor 95: 467-469.
Marchowski, D., Neubauer, G., Ławicki, Ł., Woźniczka, A., Wysocki, D. and Guentzel, S. 2015. The importance of non-native prey, the zebra mussel Dreissena polymorpha, for the declining greater scaup Aythya marila: A case study at a key European staging and wintering Site. PLoS ONE 10 (12): e0145496.
Mason, T.H.E., Stephens, P.A., Gilbert, G., Green, R.E., Wilson, J.D., Jennings, K., Allen, J.R.M., Huntley, B., Howard, C. and Willis, S.G. 2021. Using indices of species' potential range to inform conservation status. Ecological Indicators 123. DOI.
Mastrandrea, M.D., Field, C.B., Stocker, T.F., Edenhofer, O., Ebi, K.L., Frame, D.J., Held, H., Kriegler, E., Mach, K.J., Matschoss, P.R., Plattner, G.K., Yohe, G.W., and Zwiers, F.W. 2010. Guidance Note for Lead Authors of the IPCC Fifth Assessment Report on Consistent Treatment of Uncertainties. Intergovernmental Panel on Climate Change (IPCC).
Mathers, R.G., Watson, S., Stone, R. and Montgomery, W.I. 2000. A study of the impact of human disturbance on wigeon Anas penelope and Brent geese Branta bernicla hrota on an Irish Sea Loch. Wildfowl 51: 67–81.
McBlain, M., Jones, K. A. and Shannon, G. 2020. Sleeping Eurasian oystercatchers adjust their vigilance in response to the behaviour of neighbours, human disturbance and environmental conditions. Journal of Zoology 312: 75–84.
McGrady, M.J., Petty, S.J. and McLeod, D.R.A. 2004. Potential impacts of new native woodland expansion on golden eagles in Scotland. Scottish Natural Heritage Commissioned Report No. 018.
McLeod, E.M., Guay, P.J., Taysom, A.J., Robinson, R.W. and Weston, M.A. 2013. Buses, cars, bicycles and walkers: the Influence of the type of human transport on the flight responses of waterbirds. PLoS ONE, 8(12): e82008.
Meininger, P.I. and Snoek, H. 1992. Non-breeding shelduck Tadorna tadorna in the southwest Netherlands: effects of habitat changes on distribution, numbers, moulting sites and food. Wildfowl 43, 139-151.
Mendel, B., Schwemmer, P., Peschko, V., Müller, S., Schwemmer, H., Mercker, M. and Garthe, S. 2019. Operational offshore wind farms and associated ship traffic cause profound changes in distribution patterns of loons (Gavia spp.). Journal of Environmental Management 231: 429-438.
Mendel, B., Sonntag, N., Wahl, J., Schwemmer, P., Dries, H., Guse, N., Müller, S. and Garthe, S. 2008. Profiles of seabirds and waterbirds of the German North and Baltic Seas: Distribution, ecology and sensitivities to human activities within the marine environment. Bundesamt für Naturschutz, Bonn - Bad Godesberg.
Merkel F.R., Mosbech A. and Riget F. 2009. Common Eider Somateria mollissima feeding activity and the influence of human disturbances. Ardea 97, 99–107.
Merkel, F.R. and Mosbech, A. 2008. Diurnal and Nocturnal Feeding Strategies in Common Eiders Waterbirds: The International Journal of Waterbird Biology, 31, 580-586.
Mérö, T.O. and Žuljević, A. 2020. Town avenues as flight corridors for long-eared owls (Asio otus). Ornis Hungarica 28: 19-23.
Messenger, A. and Roome, M. 2007. The breeding population of the hobby in Derbyshire. British Birds 100: 594–608.
Michael, C.W. 1938. Behavior of northern phalaropes. Condor 40, 85.
Mikula, P., Díaz, M., Møller, A.P., Albrecht, T., Tryjanowski, P. and Hromada, M. 2018. Migratory and resident waders differ in risk taking on the wintering grounds. Behavioural Processes 157: 309-314.
Mikuska, T., 2009. A review of recent knowledge on white-tailed eagles in Croatia. Denisia 27: 115-126.
Miles, W.T.S. and Money, S. 2008. Behaviour and diet of non breeding snowl owls on St Kilda. Scottish Birds 28: 11-18.
Mitchell, C.R. and Hearn, R.D. 2004. Pink-footed goose Anser brachyrhynchus (Greenland/Iceland population) in Britain 1960/61 – 1999/2000. Waterbird Review Series, The Wildfowl and Wetlands Trust/Joint Nature Conservation Committee, Slimbridge.
Mitchell, J. R., Moser, M. E. and Kirby, J. S. 1988. Declines in midwinter counts of waders roosting on the Dee estuary. Bird Study 35:191-198.
Møller, A.P. 2008a. Flight distance and blood parasites in birds. Behavioral Ecology 19: 1305–1313.
Møller, A.P. 2008b. Flight distance and population trends in European birds. Behavioral Ecology 19: 1095–1102.
Møller, A.P. and Erritzøe, J. 2010. Flight distance and eye size in birds. Ethology 116: 458–465.
Møller, A.P., Nielsen, J.T. and Garamzegi, L.Z. 2007. Risk taking by singing males. Behavioral Ecology 19: 41-53.
Monteiro, L.R., Ramos, J.A. and Furness, R.W. 1996. Past and present status and conservation of the seabirds breeding in the Azores archipelago. Biological Conservation 78, 319-28.
Moore, N.P., Kelly, P.F., Lang, F.A., Lynch, J.M. and Langton, S.D. 1997. The peregrine Falco peregrinus in quarries: current status and factors influencing occupancy in the Republic of Ireland. Bird Study 44: 176-181.
Mori, Y., Sodhi, N.S., Kawanishi, S. and Yamagishi, S. 2001. The effect of human disturbance and flock composition on the flight distances of waterfowl species. Journal of Ethology 19: 115-119.
Morris, R.D. and Burness, G.P. 1992. A new procedure for transmitter attachment: effects on brood attendance and chick feeding rates by male common terns. Condor 94: 239-243.
Moss, R., Leckie, F., Biggins, A., Poole, T. and Baines, D. 2014. Impacts of human disturbance on capercaillie Tetrao urogallus distribution and demography in Scottish woodland. Wildlife Biology 20: 1–18.
Mosvi, A. H., Muneer, Y., Maher, J. A., Haider, J., Naseer, A., and Ibrahim, A. 2019. Avian Diversity Of Langh Lake Sindh And Their Response To The Disturbance., Journal of Bioresource Management 6, 9-26.
Mudge, G.P. and Talbot, T.R. 1993. The breeding biology and causes of nest failure of Scottish black-throated divers Gavia artica. Ibis 135: 113-120.
Murison, G. 2002. The impact of human disturbance on the breeding success of Nightjar Caprimulgus europaeus on heathlands in South Dorset, England. Report No. 483, English Nature, Peterborough, UK.
Natural Heritage and Endangered Species Program. 2007. Massachusetts Forestry Conservation Management Practices for Common Loons. Version 2007.1. Natural Heritage and Endangered Species Program, Massachusetts Division of Fisheries and Wildlife, Westborough, Massachusetts, USA.
NatureScot. 2013. Capercaillie Survey Methods. NatureScot guidance.
NatureScot. 2019. Concern raised over kestrel nest disturbance in the NE
Naylor, B. J. 2009. Forest management and stick-nesting birds: New direction for mitigation in Ontario. Forest Chronology 85: 235–244.
Negro, J. J. and Hiraldo, F. (1993). Nest-site selection and breeding success in the Lesser Kestrel Falco naumanni. Bird Study, 40:2, 115-119.
Nethersole-Thompson, D. 1951. The Greenshank. The New Naturalist, Collins, London.
New Hampshire Audubon. 2021. Snowy owl viewing – Observe without disturbing
Newton, I. and Campbell, C. 1973. Feeding of geese on farmland in east-central Scotland. Journal of Applied Ecology 10: 781-801.
Newton, I., Robinson, J.E. and Yalden, D.W. 1981. Decline of the merlin in the Peak District. Bird Study 8: 225-234.
Nisbet, I.C.T. 1981. Behavior of common and roseate terns after trapping. Colonial Waterbirds 4: 44-46.
Nisbet, I.C.T. 2000. Disturbance, habituation, and management of waterbird colonies. Waterbirds 23: 312–332.
Noor, A., Mir, Z.R., Khan, M.A., Kamal, A., Ahamad, M., Habib, B. and Shah, J.N. 2014. Species diversity and density of some common birds in relation to human disturbance along the bank of Dal Lake, Srinagar, Jammu and Kashmir, northwestern India. Podoces 9, 47–53.
Norris, C.A. 1945. Summary of a report on the distribution and status of the Corncrake (Crex crex). British Birds 38, 142-148, 162-16.
Norriss, D.W. and Wilson, H.J. 1988. Disturbance and flock size changes in Greenland white-fronted geese wintering in Ireland. Wildfowl 39: 63-70.
Nummi, P., Väänänen, V-M., Pakarinen, R. and Pienmunne, E. 2013. The red-throated diver (Gavia stellata) in human-disturbed habitats - building up a local population with the aid of artificial rafts. Ornis Fennica 90:16-22.
Nyatanga, T., Ndaimani, H. and Gaza, T. 2021. Local variations in the response of birds to human presence in urban areas. Ostrich doi 10.2989/00306525.2021.1976858
O'Brien, M., Green, R.E., and Wilson, J. 2006. Partial recovery of the population of Corncrakes Crex crex in Britain, 1993–2004, Bird Study 53: 213-224.
Oiseaux-Birds. 2021. Wood sandpiper
Olsen, P. and Allen, T. 1997. The trials of quarry-nesting peregrine falcons. Australian Bird Watcher 17: 87-90.
Olsen, J. and Olsen, P. 1980. Alleviating the impact of human disturbance on the breeding peregrine falcon II. Public and recreational lands. Corella 4: 54-57.
Orros, M.E. and Fellowes, M.D.E. 2014. Supplementary feeding of the reintroduced red kite Milvus milvus in UK gardens. Bird Study 61: 260-263.
OSPAR Commission. 2009. Background document for Roseate tern Sterna dougallii
Owen, M. and Williams, G.M. 1976. Winter distribution and habitat requirements of wigeon in Britain. Wildfowl 27: 83-90.
Parejo, M., Gutiérrez, J.S., Navedo, J.G., Soriano-Redondo, A., Abad-Gómez, J.M., Villegas, A., Corbacho, C., Sánchez-Guzmán, J.M. and Masero, J. 2019. Day and night use of habitats by northern pintails during winter in a primary rice growing region of Iberia. PLoS ONE 14.
Paton, D.C., Ziembicki, M., Owen, P., and Heddle, C. 2000. Disturbance distances for water birds and the management of human recreation with special reference to the Coorong region of South Australia. University of Adelaide, Adelaide, Australia. 26pp.
Paulus, S.L. 1984. Activity budgets of nonbreeding gadwalls in Louisiana. Journal of Wildelife Management 48, 371-380.
Pearce-Higgins, J.W., Finney, S. K., Yalden, D.W. and Langston, R.H.W. 2007. Testing the effects of recreational disturbance on two upland breeding waders. Ibis 2007, 149 (Suppl. 1): 45–55.
Pearce-Higgins, J.W., Stephen, L., Langston, R.H.W., Bainbridge, I.P., and Bullman, R. 2009. The distribution of breeding birds around upland wind farms. Journal of Applied Ecology 46: 1323–1331.
Pease, M.L., Rose, R.K. and Butler, M.J. 2005. Effects of human disturbances on the behavior of wintering ducks. Wildlife Society Bulletin 33, 103-112.
Penteriani, V. and Faivre, B. 2001. Effects of harvesting timber stands on goshawk nesting in two European areas. - Biological Conservation 101: 211–216.
Percival, S. 2011. Spatial and temporal patterns in black-tailed godwit use of the Humber Estuary, with reference to historic planning and development at Killingholme Pits. Report by Ecology Consulting.
Percival, S.M., Halpin, Y. and Houston, D.C. 1997. Managing the distribution of barnacle geese on Islay, Scotland, through deliberate human disturbance. Biological Conservation 82: 273-277.
Peris, S.J. and Rodriguez, R. 1997. A survey of the Eurasian kingfisher (Alcedo atthis) and its relationship with watercourses quality. Folia Zoologica 46, 33-42.
Perrow, M.R., Skeate, E.R. and Gilroy, J.J. 2011. Visual tracking from a rigid-hulled inflatable boat to determine foraging movements of breeding terns. Journal of Field Ornithology 82: 68-79.
Peters, K.A. and Otis, D.L. 2007. Shorebird roost-site selection at two temporal scales: is human disturbance a factor? Journal of Applied Ecology 44, 196–209.
Petty, S.J. 1998. Ecology and conservation of raptors in forests. Forestry Commission Bulletin 118. HMSO, London.
Pfister, C., Harrington, B.A. and Lavine, M. 1992. The impact of human disturbance on shorebirds at a migration staging area. Biological Conservation 60: 115-126.
Pienkowski, M.W. 1984. Breeding biology and population dynamics of ringed plover Charadrius hiaticula in Britain and Greenland: nest predation as a possible factor limiting distribution and timing of breeding. Journal of Zoology 202: 83-114.
Piper, W.H., Meyer, M.W., Klich, M., Tischlerd, K.B. and Dolsen, A. 2002. Floating platforms increase reproductive success of common loons. Biological Conservation 104: 199–203.
Pirovano, A., Rubolini, D., Brambilla, S. and Ferrari, N. 2000. Winter diet of urban roosting Long-eared owls Asio otus in northern Italy, the importance of the Brown rat Rattus norvegicus. Bird Study 47, 242-244.
Pitches, A. 2021. News and comment: Isle of Wight eagle reintroduction continues. British Birds 114: 643-645.
Platteeuw, M. and Henkins, R.J.H.G. 1997. Possible impacts of disturbance to waterbirds: individuals, carrying capacity and populations. Wildfowl 48, 225-236.
Prater, A. J. 1976. Breeding population of the ringed plover in Britain. Bird Study 23: 156-161.
Radović, A. and Mikuska, T. 2009. Population size, distribution and habitat selection of the white-tailed eagle Haliaeetus albicilla in the alluvial wetlands of Croatia. Biologia (Bratislava) 64: 156–164.
Rare Breeding Birds Panel. 2020a. Scaup Aythya marila.
Rare Breeding Birds Panel. 2020b. Honey buzzard survey 2020-21.
Ratchliffe, D.A. 1976. Observations on the breeding of the golden plover in Great Britain. Bird Study 23: 63-116.
Ratcliffe, D.A. 1984. Tree-nesting by peregrines in Britain and Ireland. Bird Study 31: 232-233.
Raye, L. 2021. Early modern attitudes to the ravens and red kites of London. The London Journal 2021: 1-16.
Rees, E.C., Bruce, J.H., and White, G.T. 2005. Factors affecting the behavioural responses of whooper swans (Cygnus c. cygnus) to various human activities. Biological Conservation 121, 369–382.
Reijnen, R., Foppen, R. and Meeuwsen, H. 1996. The effects of traffic on the density of breeding birds in Dutch agricultural grasslands. Biological Conservation 75, 255-260.
Richardson, C.T. and Miller, C.K. 1997. Recommendations for protecting raptors from human disturbance: a review. Wildlife Society Bulletin 25: 634–638.
Roberts, S. J., Lewis, J. M. S. and Williams, I.T. 1999. Breeding European honey buzzards in Britain. British Birds 92: 326–345.
Robson, H.J. 2017. Causes of decline of common scoter (Melanitta nigra) in north Scotland: evidence from palaeolimnology. PhD thesis, University College London.
Rodrick, E. and Milner, R. 1991. Management recommendations for Washington’s priority habitats and species. Washington Department of Wildlife, Olympia.
Rodgers, J.A. and Schwikert, S.T. 2002. Buffer-zone distances to protect foraging and loafing waterbirds from disturbance by personal watercraft and outboard-powered boats. Conservation Biology 16: 216–224.
Rodgers, J.A. and Schwikert, S.T. 2003. Buffer zone distances to protect foraging and loafing waterbirds from disturbance by airboats in Florida. Waterbirds 26: 437–443.
Rodgers, J.A. and Smith, H.T. 1995. Set-back distances to protect nesting bird colonies from human disturbance In Florida. Conservation Biology 9: 89-99.
Ross, B.P. 2000. Manipulation of feeding behaviour of diving ducks at mussel farms. PhD thesis, University of Glasgow.
Ross, K. and Liley, D. 2014. Humber Winter Bird Disturbance Study. Unpublished report for the Humber Management Scheme by Footprint Ecology.
Ross, K., Liley, D., Clarke, R., Austin, G., Burton, N., Stillman, R., Petersen, C., Panter, C., Cruickshanks, K. and Saunders, R. 2015. Habituation in wintering waterbirds. Report to Natural England.
RSPB. 1996. Action plan for the corncrake (Crex crex) in Europe.
RSPB. 2014. Calling corncrake falls silent on Rathlin
RSPB. 2015. RSPB Scotland pleased with success of osprey measures at T in the park.
RSPB. 2018. The history and future of red kite conservation
RSPB. 2021a. Impact of human activity on bird species.
RSPB. 2021b. Corncrake conservation
RSPB. 2021c. Causes of kingfisher deaths
Ruddock, M. and Whitfield, D.P. 2007. A review of disturbance distances in selected bird species. A report from Natural Research (Projects) Ltd to Scottish Natural Heritage.
Sacchi, R., Galeotti, P., Boccola, S. and Baccalini, F. 2004. Occupancy rate and habitat variables influencing nest-box use by tawny owls Strix aluco. Avocetta 28: 25-30.
Safriel, U. 1985. Diet dimorphism within an oystercatcher population – adaptive significance and effects on recent distribution dynamics. Ibis 127: 287–305.
Saga, Ø. and Selås, V. 2012. Nest reuse by goshawks after timber harvesting: Importance of distance to logging, remaining mature forest area and tree species composition. Forest Ecology and Management 270: 66–70.
Salomonsen, F. 1968. The moult migration. Wildfowl, 19, 5–24
Samia, D.S.M., Blumstein, D.T., Díaz, M., Grim, T, Ibáñez-Álamo, J.D., Jokimäki, J., Tätte, K., Markó, G., Tryjanowski, P. and Møller, A.P. 2017. Rural-urban differences in escape behavior of European birds across a latitudinal gradient. Frontiers in Ecology and Evolution 66: 1-13.
Sansom, A., Evans, R. and Roos, S. 2016. Population and future range modelling of reintroduced Scottish white-tailed eagles (Haliaeetus albicilla). Scottish Natural Heritage Commissioned Report No. 898.
Santangeli, A., Lehtoranta, H. and Laaksonen, T. 2012. Successful voluntary conservation of raptor nests under intensive forestry pressure in a boreal landscape. Animal Conservation 15: 571–578.
Scarton, F. 2018a. Flight Initiation Distances in relation to pedestrian and boat disturbance in five species of waders breeding in a Mediterranean lagoon. Revue d’Ecologie (Terre et Vie) 73: 375-384.
Scarton, F. 2018b. Disturbance of non-breeding waders by pedestrians and boats in a Mediterranean lagoon. Ardeola 65: 209-220.
Schranz, R. 2009. Effects of recreation disturbance on foraging patterns and habituation potential of Alpine wildlife: a case study of black grouse, an endangered species of timberline ecosystems. Master Thesis, University of Bern.
Schwemmer, P. Mendal, B., Sonntag, N., Dierschke, V. and Garthe, S. 2011. Effects of ship traffic on seabirds in offshore waters: implications for marine conservation and spatial planning. Ecological Applications 21: 1851-1860.
Scottish Government. n.d. Wildlife and Countryside Act 1981 Schedule 1 listed species.
Scottish Natural Heritage. 2014. Implications of Additional Protection for Hen Harrier, Red Kite and Golden Eagle under Schedules A1 & 1A of the Wildlife and Countryside Act (1981).
Scottish Natural Heritage. 2015. The use of helicopters and aircraft in relation to disturbance risks to Schedule 1 & 1A raptors and wider Schedule 1 species
Selås, V. 1997. Nest-site selection by four sympatric forest raptors in southern Norway. Journal of Raptor Research 31: 16-25.
Sergio, F. and Bogliani, G. 1999. Eurasian hobby density, nest area occupancy, diet, and productivity in relation to intensive agriculture. Condor 101: 806–817.
Sergio, F. and Bogliani, G. 2000. Hobby nest-site selection and productivity in relation to intensive agriculture and forestry. Journal of Wildlife Management 1: 637–646.
Sexton, C. 2017. Influence of the disturbance on shorebird behaviour. BSc thesis, University College Cork, Ireland.
Sharps, E., Smart, J., Mason, L.R., Jones, K., Skov, M.W., Garbutt, A. and Hiddink, J.G. 2017. Nest trampling and ground nesting birds: Quantifying temporal and spatial overlap between cattle activity and breeding redshank. Ecology and Evolution. DOI: 10.1002/ece3.3271.
Shawyer, C. R. 2011. Barn Owl Tyto alba survey methodology and techniques for use in ecological assessment: developing best practice in survey and reporting. IEEM, Winchester.
Shepherd, P.C.F. and Lank, T.B. 2004. Marine and agricultural habitat preferences of dunlin wintering in British Columbia. Journal of Wildlife Management 68: 61-73.
Sinnott, R. 2000. Effects of recreational trails and related human activities on birds: An annotated bibliography. Alaska Department of Fish and Game, Division of Wildlife Conservation, Anchorage.
Slankard, K. G., Taylor, L.F., Stoelb, D.M. and Gannon, C. 2020. Peregrine falcons nest successfully during reconstruction of bridge over Ohio River. Human–Wildlife Interactions 14(1).
Smit, C.J. and Visser, G.J.M. 1993. Effects of disturbance on shorebirds: a summary of existing knowledge from the Dutch Wadden Sea and Delta area. Wader Study Group Bulletin 68: 6–19.
Snow, D.W. and Perrins, C.M. (eds) 1998. The Birds of the Western Palearctic, Concise edition. London Oxford University Press.
Solheim, R. 2021. Snowy owl hunting behaviour and prey spotting distances revealed by vole lures. Airo 29: 460-466.
Solheim, R., Øien, I.J. and Aarvak, T., Jacobsen, K.O. 2021. Snowy Owl (Bubo scandiacus) males select the highest vantage points around nests - WOC 2017. Airo 29: 451-459.
South of Scotland Golden Eagle Project. 2021. South of Scotland Golden Eagle Project.
Spaans, B., Leopold, M. and Plomp, M. 2018. Using a drone to determine the number of breeding pairs and breeding success of Sandwich terns Sterna sandvicensis. Limosa 91: 30-37.
Spaul, R. J. and Heath, J.A. 2017. Flushing responses of golden eagles (Aquila Chrysaetos) in response to recreation. The Wilson Journal of Ornithology 129: 834-845.
Stalmaster, M.V. and Kaiser, J.L. 1997. Effects of recreational activity on wintering bald eagles. Wildlife Monographs 137: 5-46.
Stanbury, A., Eaton, E., Aebischer, N., Balmer, D., Brown, A., Douse, A., Lindley, P., McCulloch, N., Noble, D. and Win, I. 2021. The status of our bird populations: the fifth Birds of Conservation Concern in the United Kingdom, Channel Islands and Isle of Man and second IUCN Red List assessment of extinction risk for Great Britain, British Birds, 114 (December 2021), pp. 723–747.
Stanevicius, V. 2004. Nest site selection by marsh harrier (Circus aeruginosus) in the shore belt of helophytes on large lakes. Acta Zoologica Lituanica 14: 47–53.
Stein, J. and Ims, R.A. 2016. Absence from the nest due to human disturbance induces higher nest predation risk than natural recesses in common eiders Somateria mollissima. Ibis 158, 249–260.
Stillman, R. A. and Goss-Custard, J. D. 2002. Seasonal changes in the response of oystercatchers Haematopus ostralegus to human disturbance. Journal of Avian Biology 33: 358-365.
Storch, I. 2000. Status Survey and Conservation Action Plan 2000-2004 Grouse. - WPA/BirdLife/SSC Grouse Specialist Group. IUCN, Gland, Schweiz und Cambridge,UK und World Pheasant Association, Reading, UK.
Strasser, E.H. 2010. Reproductive failure and the stress response in American kestrels nesting along a human disturbance gradient. PhD thesis, Boise State University.
Strasser, E.H. and Heath, J.A. 2013. Reproductive failure of a human-tolerant species, the American kestrel, is associated with stress and human disturbance. Journal of Applied Ecology 50, 912-919.
Stroud, D.A., Fox, A.D., Urquhart, C. and Francis, I.S. 2012. International Single Species Action Plan for the Conservation of the Greenland White-fronted Goose (Anser albifrons flavirostris). AEWA Technical Series No. 45. Bonn, Germany.
Sultana, M. and Sarker, N. J. 2016. Patterns of daily activities frequencies of Common Kingfisher (Alcedo atthis) in Nikunja-1, Dhaka. International Journal of Fauna and Biological Studies 3, 23-28.
Summers, R., Mavor, R. and Hogg, S. 1994. Factors affecting loch selection and breeding success of Slavonian grebes in Scotland. Report to Scottish Natural Heritage.
Summers, R.W. 2018. Foraging patterns of common crossbills (Loxia curvirostra) on spruces (Picea spp.) in Scotland. Forestry 91: 444–450.
Summers, R.W., Mavor, R.A., Buckland, S.T. and MacLennan, A.M. 1999. Winter population size and habitat selection of Crested Tits Parus cristatus in Scotland, Bird Study, 46:2, 230-242.
Summers, R.W., Mavor, R.A., Hogg, S. and Harriman, R. 2011. Lake characteristics and their selection by breeding Slavonian grebes Podiceps auritus in Scotland. Bird Study 58: 349-356.
Summers, R. W., McFarlane, J. and Pearce-Higgins, J.W. 2007. Measuring avoidance by capercaillies Tetrao urogallus of woodland close to tracks. Wildlife Biology 13: 19–27.
Sunde, P., Odderskær, P. and Storgaard, K. 2009. Flight distances of incubating common buzzards Buteo buteo are independent of human disturbance. Ardea 97: 369–372.
Sutton, G. M. and Parmelee, D. F. 1956. Breeding of the Snowy Owl in Southeastern Baffin Island. Condor 58: 273-282.
Svensson, L., Mullarney, K., Zetterström, D., Grant, P.J. and Christie, D.A. 2009. Collins bird guide, 2nd edition. Harper Collins, London.
Swenson, J.E. 1979. Factors affecting status and reproduction of ospreys in Yellowstone National Park. Journal of Wildlife Management 43: 595-601.
Tapia, L., Dominguez, J. and Rodriguez, L. 2004. Modelling habitat use and distribution of hen harriers (Circus cyaneus) and Montagu's harrier (Circus pygargus) in a mountainous area in Galicia, north-western Spain. Journal of Raptor Research 38: 133-140.
Taylor, D.P., Dvorett, D.A., Vradenburg, J.A. and Smith, L.M. 2019. Influence of Environmental Stress and Anthropogenic Disturbance on the Energy Expenditure of Wintering Northern Pintails (Anas acuta). Waterbirds 42, 294-303.
Taylor, I.R. 2006. Managing visitor disturbance of waterbirds on Australian inland wetlands. Pages 150–157 in Taylor IR, Murray PA, Taylor SG, editors. Wetlands of the Murrumbidgee River Catchment: Practical Management in an Altered Environment. Fivebough and Tuckerbil Wetlands Trust, Leeton, New South Wales.
Thiel, D., Ménoni, E., Brenot, J. and Jenni, L. 2007. Effects of recreation and hunting on flushing distance of capercaillie. Journal of Wildlife Management 71: 1784–1792.
Thom, V.M. 1986. The Birds of Scotland. T and AD Poyser Ltd, London.
Tierney, N., Whelan, R. and Valentin, A. 2016. Post-breeding aggregations of roosting terns in south Dublin Bay in late summer. Irish Birds 10: 339-344.
Titus, J.R. and VanDruff, L.W. 1981. Response of the common loon to recreational pressure in the Boundary Waters canoe area, north-western Minnesota. Wildlife Monographs 79: 5-59.
Tjørve, K.M.C. and Tjørve, E. 2010. Contrasting reactions to human disturbance in Eurasian Oystercatchers Haematopus ostralegus on the south coast of Norway. Wader Study Group Bulletin 117: 62.
Topping, C. and Petersen, I.K. 2011. Report on a red-throated diver agent-based model to assess the cumulative impact from offshore wind farms. Report commissioned by Vattenfall A/S. Aarhus University, DCE - Danish Centre for Environment and Energy.
Tratalos, J.A., Gill, J.A., Jones, A., Showler, D., Bateman, A., Watkinson, A., Sugden, R. and Sutherland, W. (2005). Interactions between tourism, breeding birds and climate change across a regional scale. Tyndall Centre Technical Report 36. Tyndall Centre for Climate Change Research.
Tratalos, J.A., Jones, A.P., Showler, D.A., Gill, J.A., Bateman, I.J., Sugdene, R., Watkinson, A.R. and Sutherland, W.J. 2021. Regional models of the influence of human disturbance and habitat quality on the distribution of breeding territories of common ringed plover Charadrius hiaticula and Eurasian oystercatcher Haematopus ostralegus. Global Ecology and Conservation 28, e01640, 08.2021.
Trulio, L.A. and White, H.R. 2017. Wintering waterfowl avoidance and tolerance of recreational trail use. Waterbirds 40: 252-262.
Tuite, C. H., Hanson, P. R. and Owen, M. 1984. Some ecological factors affecting winter wildfowl distribution on inland waters in England and Wales, and the influence of water-based recreation. Journal of Applied Ecology 21, 41-62.
Upton, A.G., Williams, S.J. and Williams, E.J. 2018. North Orkney proposed Special Protection Area (pSPA): Inshore wintering waterfowl survey 2017/18. Scottish Natural Heritage Commissioned Report No. 1074.
Urfi, A.J., Goss-Custard, J.D. and Le V. dit Durell, S.E.A. 1996. The ability of oystercatchers Haematopus ostralegus to compensate for lost feeding time: Field studies on individually marked birds. Journal of Applied Ecology 33: 873-883.
Urquhart, C., Fox, A.D., Francis, I., Griffin, L., Mitchell, C. and Stroud, D.A. 2015. Greenland white-fronted goose. Version 1.1. In: The Species Action Framework Handbook, Gaywood, M.J., Boon, P.B., Thompson, D.B.A. and Strachan, I.M. (eds). Scottish Natural Heritage, Battleby, Perth.
Urquhart, G. 2011. Northern Harrier (Circus cyaneus). Michigan Breeding Bird Atlas II 1, 1-4.
U.S. Fish and Wildlife Service. 1991. Northern harrier habitat model
Väänänen, V-M. 2001. Hunting disturbance and the timing of autumn migration in Anas species. – Wildlife Biology 7, 3–9.
Valle, R.G. and Scarton, F. 2021. Drone-conducted counts as a tool for the rapid assessment of productivity of Sandwich terns (Thalasseus sandvicensis). Journal of Ornithology 162: 621-628.
van de Pol, M., Ens, B.J., Oosterbeek, K., Brouwer, L., Verhulst, S., Tinbergen, J.M., Rutten, A.L. and de Jong, M. 2009. Oystercatchers’ bill shapes as a proxy for diet specialization: more differentiation than meets the eye. Ardea 97: 335–347.
van der Horst, S., Goytre, F., Marques, A., Santos, S., Mira, A. and Lourenco, R. 2019. Road effects on species abundance and population trend: a case study on tawny owl. European Journal of Wildlife Research 65: 99.
van Dijk, K. 2014. Notes on the foraging behaviour of Eurasian oystercatchers Haematopus ostralegus feeding on bread. Wader Study Group Bulletin 121: 15–17.
van Gompel, J. 1979. The occurrence of the short-eared owl Asio flammeus on the Belgian coast. Gerfaut 69: 83-110.
Vas, E., Lescroël, A., Duriez, O., Boguszewski, G. and Grémillet, D. 2015. Approaching birds with drones: first experiments and ethical guidelines. Biological Letters 11: 20140754–20140754
Verhulst, S., Oosterbeek, K. and Ens, B.J. 2001. Experimental evidence for effects of human disturbance on foraging and parental care in oystercatchers. Biological Conservation 101: 375–380.
Versluijs, M. 2011. Afro-Siberian bar-tailed godwits on inland coastal meadows during spring migration. Professional bachelor thesis: Department of Marine Ecology. NIOZ Royal Netherlands Institute for Sea Research.
Vincze, E., Papp, S., Preiszner, B., Seress, G., Bokony, V. and Liker, A. 2016. Habituation to human disturbance is faster in urban than rural house sparrows. Behavioral Ecology 27: 1304-1313.
Virzi, T. 2010. The effect of human disturbance on the local distribution of American Oystercatchers breeding on barrier island beaches. Wader Study Group Bulletin 117: 19–26.
Wallgren, H. 2003. Nest visibility- no trend over 27 years despite changed behaviour of the eagles. Sea Eagle 2000. Proceedings from the International Sea Eagle Conference pp 371-375.
Wallis, K., Hill, D., Wade, M., Cooper, M., Frost, D. and Thompson, S. 2019. The effect of construction activity on internationally important waterfowl species. Biological Conservation 232: 208–216.
Waterhouse, F.L. and Harestead, A.S. 1999. The Value of Riparian Forest Buffers as Habitat for Birds in the Coastal Western Hemlock Zone, British Columbia, a Pilot Study. Technical Report 001 September 1999 Research Section, Vancouver Forest Region, BCMOF 16 TR-001 Wildlife September 1999.
Watson, A. 1957. The behaviour, breeding, and food ecology of the Snowy Owl (Nyctea scandiaca). Ibis 99: 419-462.
Watts, B.D., Smith, F.M., Hines, C., Duval, L., Hamilton, D.J., Keyes, T., Paquet, J., Pirie-Dominix, L., Rausch, J., Truitt, B., Winn, B. and Woodard, P. 2021. The costs of using night roosts for migrating whimbrels. Journal of Avian Biology 52.
Wernham, C.V., Toms, M.P., Marchant, J.H., Clark, J.A., Siriwardena, G.M. and Baillie, S.R. (eds). 2002. The Migration Atlas: movements of the birds of Britain and Ireland. T. and A.D. Poyser, London.
West, A., Goss–Custard, J., Stillman, R., Caldow, R., Durell, S. and McGrorty, S. 2002. Predicting the impacts of disturbance on shorebird mortality using a behaviour–based individuals model. Biological Conservation 106: 319–328.
Weston, M.A., McLeod, E.M., Blumstein, D.T. and Guay, P-J. 2012. A review of flight-initiation distances and their application to managing disturbance to Australian birds. Emu 112: 269–286.
Weston, M.A., Radkovic, A., Kirao, L., Guay, P-J., van Dongen, W.F.D., Malaki, P., Blumstein, D.T. and Symonds, M.R.E. 2021. Differences in flight initiation distances between African and Australian birds. Animal Behaviour 179: 235-245.
White, D.M. and Sherrod, S.K. 1973. Advantages and disadvantages of the use of rotor-winged aircraft in raptor studies. Raptor Research 7: 97–104.
Whitfield, D.P., Macleod, D.R.A., Watson, J., Fielding, A.H. and Haworth, P.A. 2003. The association of grouse moor in Scotland with the illegal use of poisons to control predators. Biological Conservation 114: 157-163.
Whitfield, D.P., Ruddock, M. and Bullman, R. 2008a. Expert opinion as a tool for quantifying bird tolerance to human disturbance. Biological Conservation 141: 2708-2717.
Whitfield, D.P., Fielding, A.H., and Whitehead, S. 2008b. Long-term increase in the fecundity of hen harriers in Wales is explained by reduced human interference and warmer weather. Animal Conservation 11: 144–152.
Whitfield, D.P., Fielding, A.H., Gregory, M.J.P., Gordon A.G., McLeod, D.R.A. and Haworth, P.F. 2007. Coplex effects of habitat loss on golden eagles Aquila chrysaetos. Ibis 149: 26-36.
Whitfield, D. P. and Rae, R. 2014. Human disturbance of breeding Wood Sandpipers Tringa glareola: Implications for “alert distances” in prescribing protective buffer zones. - Ornis Fennica 91: 57–66.
Wiklund, C. G., and Stigh, J. 1983. Nest Defence and Evolution of Reversed Sexual Size Dimorphism in Snowy Owls Nyctea scandiaca. Scandinavian Journal of Ornithology 14: 58–62.
Wildman, L., O’Toole, L. and Summers, R.W. 1998. The diet and foraging behaviour of the red kite in northern Scotland. Scottish Birds 19: 134-140.
Wilke, A.L., and Johnston-González, R. 2010. Conservation Plan for the Whimbrel (Numenius phaeopus). Version 1.1. Manomet Center for Conservation Sciences, Manomet, Massachusetts.
Williamson, K. 1947. The distraction display of the ringed plover, Charadrius hiaticula hiaticula Linnæus. Ibis 89: 511-513.
Wilson, K.A. 1938. Owl studies at Ann Arbor, Michigan. Auk 55: 187-197.
Wilson, W.M., Balmer, D.E., Jones, K., King, V.A., Raw, D., Rollie, C.J., Rooney, E., Ruddock, M., Smith, G.D., Stevenson, A., Stirling-Aird, P.K., Wernhama, C.V., Weston, J.M. and Noble, D.G. 2018. The breeding population of Peregrine Falcon Falco peregrinus in the United Kingdom, Isle of Man and Channel Islands in 2014. Bird Study 65: 1-19.
Woodward, I., Aebischer, N., Burnell, D., Eaton, M., Frost, T., Hall, C., Stroud, D.A. and Noble, D. 2020. Population estimates of birds in Great Britain and the United Kingdom. British Birds 113: 69–104.
Woodward, I.D., Ross-Smith, V.H., Pérez-Dominguez, R., Rehfisch, M. and Austin, G.E. 2015. The Wash bird decline investigation 2014. BTO Research Report No. 660.
Yalden, P.E. and Yalden, D.W. 1990. Recreational disturbances of breeding golden plovers Pluvialis apricarius. Biological Conservation 51: 243–262.
Yalden, D.W. and Yalden, P.E. 1989. The sensitivity of breeding golden plovers Pluvialis apricaria to human intruders. Bird Study 36: 49-55.
Zeitler, A. 2000. Human disturbance, behaviour and spatial distribution of black grouse in skiing areas in the Bavarian Alps. Cahiers d’Ethologie 20: 381-402.




