Research Report 1292 - Towards understanding the effectiveness of measures to manage fishing activity of relevance to MPAs in Scotland
Year of publication: 2024
Authors: Sarah Cunningham, David Donnan, Katie Gillham, Ben James, Lisa Kamphausen and Suzanne Henderson (NatureScot), Peter Chaniotis and Eirian Kettle (JNCC) and Phil Boulcott and Peter Wright (Marine Scotland Science)
Cite as: Cunningham, S., Donnan, D., Gillham, K., James, B., Kamphausen, L., Henderson, S., Chaniotis, P., Kettle, E., Boulcott, P., and Wright, P. 2024. Towards understanding the effectiveness of measures to manage fishing activity of relevance to MPAs in Scotland. NatureScot Research Report 1292.
Introduction
Background to MPA designation and management
Marine Protected Areas (MPAs) are a globally recognised tool to support conservation of the marine environment. In Scotland MPAs have generally been established to either maintain the condition of the features they have been designed to protect or to help them recover. There are international commitments relating to creating and effectively managing MPAs for habitats and species, e.g. Convention on Biodiversity, OSPAR Convention for the North-East Atlantic and the EU Habitats and Birds Directives.
When MPAs are designated management measures are normally implemented through legislation, voluntary measures or codes of best practice to help achieve the conservation objectives of the protected features. Management measures in MPAs vary depending on the activities present, the sensitivities of the protected features to the pressures created by activities and the policies in the country/area. MPAs are expected to conserve the protected species or habitats contained within them and have wider ecosystem benefits. Most MPAs in Scotland have been established to protect a part or component of the wider ecosystem; namely specifically listed habitats, species, geological features or large scale features/processes. Under this approach, some activities may continue to operate as long as they do not compromise the achievement of the conservation objectives for the protected features. This is often achieved through spatial or temporal management measures (as is the case for example in the management of specific fishing activities) and / or through wider mitigation approaches, such as changing the method or infrastructure used by an activity (as is the case under the marine licensing process for renewables, oil and gas extraction and aquaculture).
Impacts of fishing in the marine environment
Commercial fishing activity can impact both benthic habitats and species as well as more mobile species. The Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES, 2019) concluded that human activities have had a large and widespread impact on the world’s oceans. These include direct exploitation, in particular overexploitation, of fish, shellfish and other organisms, and that this has had the largest relative impact on the marine environment over the past 50 years. The impact of different fishing gears on marine habitats and species is becoming increasingly well understood and is underpinned by a substantial and growing scientific evidence base (Hiddink et al., 2017, 2019; Kaiser et al., 2006, Kaiser et al., 2018; Rijnsdorp et al., 2018; Sciberras et al., 2018). The most recent UK Marine Strategy Assessment (Defra, 2019) concluded that physical disruption of the seabed from fishing gear, in particular the use of certain types of demersal gear, is a key factor preventing achievement of good environmental status (GES) for some seabed habitats. Scotland’s Marine Assessment 2020 highlighted pressures associated with bottom contacting and pelagic fishing as being the most widespread, direct pressures across the majority of Scottish Marine Regions and Offshore Marine Regions.
Approach to managing fishing activity within MPAs in Scotland
There is a presumption of multiple-use within MPAs in Scotland but it is recognised that various activities, including different types of fishing, are capable of affecting the protected features markedly and may require management. The evidence for the sensitivity of features in MPAs to various activities, including fishing, is set out in the Feature Activity Sensitivity Tool (FeAST) and Fisheries Guidance Notes. NatureScot and JNCC provide management advice for MPAs that focusses on fishing activities that cause an effect (a pressure) that the protected features are sensitive to. It takes a risk-based approach and focusses on activities that have the potential to pose a risk to achieving the conservation objectives for the protected features. Marine Scotland considers whether management measures need to be implemented based on this advice. Marine Scotland lead on the development of fisheries management measures and work is ongoing with stakeholders to develop and implement measures for MPAs in territorial waters. In Scottish offshore waters MPA measures will now be developed and agreed under national legislation following the UK’s exit from the European Union.
Fisheries restrictions also exist for reasons other than nature conservation in Scotland, including commercial species management or by default through other activities such as the development of offshore windfarms.
Reviewing the effectiveness of management in MPAs
Reviewing and assessing the effectiveness of management implemented for MPAs can be challenging due to the factors outlined further in this paper. This paper does not set out to formally assess the effectiveness of measures implemented for managing fishing activity in relation to MPAs and other areas. Rather, it brings together relevant case studies in one place, discuss the outcomes and develop conclusions from these of relevance to MPA management for Scotland.
Aims of paper
The purpose of this paper is to outline current evidence from within the UK and other temperate regions regarding the efficacy of fisheries management measures in safeguarding the protected features of MPAs. It also captures any wider biodiversity benefits that have been noted. This paper is divided into three main sections that explore:
- case studies of MPAs with fisheries management measures for a biodiversity benefit (although results reported are largely related to commercial species);
- spatial management measures for fisheries (objective is fisheries related) or for other purposes;
- planned work in Scotland that will provide additional evidence and areas for further development and consideration.
It should be noted that this paper does not aim to provide a comprehensive analysis of all the literature available on this subject, but rather presents a snapshot of the evidence we are most familiar with and have utilised previously in providing advice. We recognise that there is likely to be additional evidence available and that this is likely to build in the future as MPAs and their management measures become more mature. We will aim to update this paper in advance of the Report to the Scottish Parliament on Marine Protected Areas (as required under the Marine (Scotland) Act 2010) which is undertaken every 6 years (first publication December 2018).
Challenges for assessing the effectiveness of MPA management
Environmental challenges
Determining if MPA management measures have been effective in supporting MPAs to achieve their stated objectives is challenging, especially if attempted over relatively short time periods e.g. a few years. The sea is a highly dynamic environment, where environmental conditions and some populations of species fluctuate naturally from year to year. There are also significant long-term drivers of environmental change such as climate change that influence every element of the ecosystem in ways which over short time scales are often chaotic and relatively unpredictable. Detecting trends against this background of high variability generally requires data collection over many years, ideally decades. To then attribute these trends to causes, such as introduction of specific management measures, adds another layer of complexity. Nevertheless, in the short term, an assessment on the level of compliance with management measures and the inferred reduction on pressure resulting from this action can be useful (Langton et al., 2020).
Biological challenges
The slow growth of many marine species and habitats also hampers short-term assessments of management efficacy. For example, maerl grows in the region of 1 mm per year so a change in the density of live maerl on a bed (as a metric of habitat health) might take 25 years or more to discern. An assessment made only a few years after the introduction of measures must instead rely on other indicators. These may show us that pressures have been eased sufficiently to enable recovery or halt decline in the entire system although the relationships between some indicators and the response of species and habitats are not fully understood. Many marine species also reproduce at irregular intervals. It is not uncommon for five or more years to pass between large recruitment events. Therefore, whilst the effects of fisheries management measures may not be apparent in the short term this does not mean the measures aren’t effective. Further complication is added when considering spatial scales (Boulcott et al., 2018). Most MPAs are relatively small, while most marine organisms reproduce via highly dispersive larval or propagule life stages, which can be carried large distances before settling (Millar et al., 2019). Recruitment to a patch within a MPA may therefore often be dependent on larval supply from outside the site. Similarly, where protected areas offer nursery habitats for juveniles of species that are more mobile as adults, the effect of an increase in the stock of adults would not be detectable in the same place as the protected nursery habitat.
Quantifying and communicating wider benefits
Most MPAs in Scotland afford protection to specific features, mainly habitats and species. There is a growing recognition of the range of ecosystem services provided by MPA features e.g. nursery habitats, coastal protection, carbon sequestration etc. but to date the focus of monitoring has largely been on determining the status of the designated interests themselves rather than the services they provide. Better quantification of societal benefits or socio-economic returns arising from fisheries management measures, to demonstrate offsetting of economic ‘costs’ incurred (in terms of restricting activity) is required but good metrics are currently lacking and is unlikely to be adequately informed by existing monitoring programmes.
Management of fishing activity within MPAs
Overview
This section provides examples of MPAs where fisheries management measures have been put in place for the purpose of biodiversity conservation. Various overview papers (e.g. Huijibers et al., 2015, Lemasson et al., 2019) focus on the benefits that have been seen in relation to commercial fish and shellfish stocks. Lemasson et al., (2019) published a review of studies where fisheries management of varying degrees has been put in place in MPAs. This covered both temperate and tropical examples ranging from full No Take Zones (NTZs) to various fishing gear restrictions. Huijibers et al., (2015) noted that 150 studies published between 1977 and 2012 of MPAs prohibiting all fishing (no-take areas/reserves) across the world found that they had more invertebrates compared to fished areas outside (Huijibers et al., 2015). Similarly Sciberras et al., (2018) undertook a review of 27 studies published before February 2011 of MPAs where fishing had been partially restricted and found that they had greater abundances of scallops and lobsters compared to outside the MPA boundaries.
There is a lack of evidence in the results of the studies covered in these papers about the effects of reducing or removing fishing pressure in MPAs on wider biodiversity, in particular benthic habitats and non-commercial species. This is in part due to the challenges described in section 1.2 and a general lack of long-term studies in MPAs, especially in temperate waters. The following case studies presented here are summarised from the information in these review papers and evidence from other sources. They provide a synopsis of the most relevant information from available case studies of most relevance to MPAs in Scotland. The case studies are presented in the following sections, starting with those from Scotland, the rest of the UK and finally those from other temperate countries. A summary table of the key information and conclusions from these case studies is provided in Appendix 1.
Darwin Mounds SAC, Scotland
Background
The Darwin Mounds were first discovered in 1998 during a seabed survey undertaken for the Atlantic Frontier Environment Network (AFEN). The Mounds lie approximately 160 km north-west of Cape Wrath, Scotland at a depth range of approximately 700 - 1,100 m. It was, at the time, the only example of Lophelia pertusa (cold-water coral) reef colonies found growing in ‘thickets’ from sandy seabed substrate (Figure 1).
Upon finding evidence of significant physical damage at the site in 2000, a request for a temporary closure was made to the European Commission by the UK Government in 2003, which was made permanent in 2004 under the EC Regulation 602/2004. The site was designated as a Special Area of Conservation (SAC), under the European Commission Habitats Directive, in 2008.
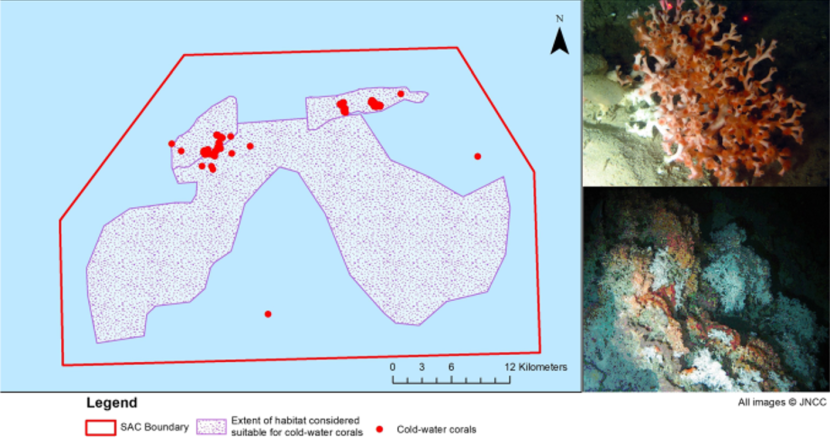
Three images are shown. The boundary of the Darwin Mounds SAC is shown in the left hand image within which is the extent of the habitat suitable for cold water corals to grow and red dots mostly in the north of this area show where cold water corals have been found. The two images to the right of the map show cold water corals growing on sediment and rock.
Review of effectiveness
The current condition of the site is considered to be unfavourable, and thus the features need to be restored to favourable condition. The analysis of the 2011 survey (JC060) data indicated that a significant reduction in trawling intensity took place between 2003 and 2011. The reduction was particularly marked in the eastern area of the Darwin Mounds, the area considered most heavily impacted in 2000, indicating that the bottom-trawling closure is largely being respected (Huvenne et al., 2016).
However, these results also indicate that there has been very little recovery of the coral community between implementation of the fisheries closure in 2003 and the survey that took place in 2011 (Chaniotis et al., 2019). Whilst the closure has been effective in reducing fishing pressure on the cold-water coral reefs and in preventing further damage to the feature, there have been no signs of reef recovery since the fisheries closure was brought into force (Huvenne et al.,2016). While the reason for the absence of recovery is unknown, it might be linked with life history factors such as reproduction, larval dispersal and connectivity. Indeed, Lophelia pertusa at the Darwin Mounds SAC does not appear to exhibit sexual reproduction, displaying a high number of genetic clones and likely low recruitment rates of sexually produced larvae (Le Goff‐Vitry et al., 2004; Waller & Tyler 2005 in Chaniotis et al., 2019). It appears that larval recruitment for recovery might be reliant on immigration, with larval supply to the Darwin Mounds SAC shown to be predominantly derived from Rosemary Bank Seamount (Ross et al.,2017). In addition work by Dourain et al., (2013) indicates that there appears to be a hiatus in cold-water coral growth in the North-east Atlantic related to larger- scale shifts in circulation and this may also be a compounding factor. There has been additional survey work in site in 2019 and the results should be available soon.
Lamlash Bay No Take Zone, Scotland
Background
Lamlash Bay No Take Zone (NTZ) was established in 2008 covering 2.67 km2 of the seabed off the east coast of the Isle of Arran. It was Scotland’s first NTZ and was the result of a campaign led by the Community of Arran Seabed Trust (COAST). Stewart et al., (2020) provide a summary of the background to the NTZ. The COAST campaign for the area was fuelled by the decline in fin fish populations in the bay and the Clyde more widely, and by the impact of trawling and dredging activity on sensitive marine habitats in the area. Lamlash Bay contains one of the densest remaining known maerl bed patches in the Clyde, small seagrass beds, kelp and rocky reefs. The NTZ was put in place to look at the potential for regeneration of marine life in the bay and it prohibits the removal of all fish and shellfish from the waters and seabed including the intertidal area. For context in relation to the studies outlined below, the South Arran MPA was designated in 2014 and it includes the NTZ. Management measures for fishing activity across the South Arran MPA came into effect in 2016 and include prohibition of scallop dredging and trawling across a much wider area encompassing the protected features.
Review of effectiveness
The NTZ benefits from a high degree of engagement and awareness in the local community, with COAST also being engaged in surveillance and monitoring. This has helped to support compliance with the management measures although there have been concerns about the risk of intrusions into the NTZ. However, in general, there is no exploitation of biological resources and there is little or no benthic disturbance arising from other activities within the NTZ.
The small size of the NTZ and its still relatively young age make it particularly difficult to define measurable biological effects for some species and at present the data do not yet paint a consistent picture, although newer data for benthic habitats is starting to show positive signs. Student research projects conducted in the NTZ by the University of York since 2010 have generated a range of data on specific metrics. These studies and data are described in further detail below.
Findings by research projects include significantly larger and older king scallops (Pecten maximus) inside the NTZ than outside (Howarth et al., 2015a). The abundance of juvenile scallops was also found to be significantly greater within the NTZ compared to outside, and this was correlated with a greater presence of macroalgae and hydroids inside the NTZ (Howarth et al., 2015a). However, changes in abundance may take more time. A Marine Scotland Science study investigated the effects of the NTZ on the abundance of adult Pecten maximus and Aequipecten opercularis by comparing data collected during large-scale photographic surveys inside and outside the NTZ in 2009, 2010 and 2014 (Boulcott et al., 2018). Although the abundance of scallops varied over the three years of the study, no significant effect of the protection status or of the interaction between protection status and year was found. This matches findings of a previous study by the University of York in 2010, which also found no significant effect of the NTZ on adult scallop densities (Howarth et al., 2015a).
More recently James (2019) undertook diver surveys of scallops inside and outside the NTZ and South Arran MPA in the same locations used previously with some additional sites. The study found significant increases of between 3.4 and 6.2 times the density of legal sized king scallops in the NTZ and locations within the MPA (locations were near to and further away from the NTZ). King scallop density increased significantly (10-fold) in the period 2014-2019 in the MPA (outside of the NTZ) since designation and implementation of associated fisheries measures. The density of scallops was significantly higher in the NTZ compared to fished sites and there were more legal-sized, larger scallops, with a significant difference in age classes between the fished areas (younger) and NTZ (older). Gonad size was also significantly greater in scallops within the NTZ. However, for juvenile scallops, their abundance has fluctuated in all locations and no significant differences were detected amongst the locations, which could be due to the variations in the pattern of recruitment across the area. The presence of bryozoans and macroalgae was shown to be significantly correlated with the abundance of juvenile scallops, supporting previous studies that suggest they settle on more complex benthic flora (Kamenos et al. 2004).
For other species the picture is varied, and no difference was found in the abundance and size of fish (Howarth et al., 2015a). Dyman (2022) reviewed crustacean data for all survey years (2012-2022 excluding 2016 and 2019 when no surveys were conducted) for the NTZ in comparison to control areas (near and far from the NTZ but within the demersal mobile gear closure area of the South Arran MPA). These data show variability in catch per unit effort (CPUE) over time for all species examined (lobster, brown crab and velvet crab). This supports previous findings that noted the variability in CPUE for velvet crab but differs from studies that showed catches were higher in the NTZ for brown crab and lobster (Howarth et al., 2016; Stewart et al., 2020). These previous studies used a shorter time span of data to examine differences. Results for 2020, 2021 and 2022 have shown changing trends in abundance of lobsters (CPUE) compared to the earlier data (2010-2018). In 2022 CPUE and also weight per unit effort (WPUE), was greater in the NTZ than the near control area. But CPUE and WPUE were highest overall in the far control areas, which also had the highest percentage of lobsters below legal landing size. However, mean size of lobsters and the percentage of legal sized lobsters was highest in the NTZ compared to near and far control areas (both of which are within the MPA), supporting previous conclusions by Howarth et al., (2016). From 2016 to 2022, mean lobster size was consistently lower in the far control than in the NTZ. Lobsters with a mean size greater or equal to 111mm were only found in the NTZ and near control.
CPUE as an indicator of abundance is useful to have but abundance should not be seen as the only indicator of ‘success’ of a management measure. The increased size of the lobsters in the site is a significant measure in an ecological sense - i.e. previous lobster research has indicated that larger lobsters produce better quality and more eggs compared to smaller lobsters (Hepper & Gough, 1978).
Dyman (2022) suggests that the variation seen in the time series of data could be due to a variety of factors such as climatic changes, interspecific interactions and survey design factors (location, bait used for creels). Dyman (2022) also noted that higher lobster catch rates showed a moderately strong inverse correlation with velvet crabs and brown crabs. The inverse correlation between lobsters and brown crabs in particular is an indication of competition between the species. In addition because the control areas within the MPA have been protected from the impacts of bottom-towed fishing gear since 2016, it is not known how this, and any associated recovery of seabed habitats may be influencing crustacean recruitment and survival. Finally, there is also likely to be variation in fishing intensity, with Dyman (2022) suggesting that the lower CPUE of legal-sized lobsters in the near control may also be attributable to “fishing the line,” where fishers are active close to the border of a protected area to take advantage of spillover. No fishing activity/intensity data were available to examine this further. Previous tag recapture studies have indicated that individuals are moving from within the NTZ to areas outside (Howarth et al., 2016; Crimmins, 2018). There was limited evidence of this in the recapture analysis by Dyman (2022) - only one of the recaptured lobsters moved from one treatment area to another (NTZ to near control) and all other recaptured lobsters (14) were found in the same area as they had previously been tagged (i.e. NTZ, or near/far control).
In relation to seabed habitats, a NatureScot dive survey in 2014 studied the maerl bed in Lamlash Bay and recorded 55-75% live maerl coverage (Mercer et al., 2018). The bed represents a good example of the habitat in the region; dense maerl beds are now only known to occur in very few isolated patches in the Clyde. However it is not possible from the available evidence to determine the effect of the NTZ on the maerl bed’s extent and condition. No pre-NTZ data is available to make more detailed comparisons of changes to the bed after designation of the NTZ but given the slow growth rate of maerl we would not have expected to see much development between designation in 2008 and the survey in 2014. Maerl beds are highly sensitive to scallop dredging (Hall-Spencer and Moore, 2000) and its presence at the time of designation and its location (within depth contours which would make it awkward to access by dredge) suggests that it may not have been disturbed by fishing prior to the implementation of the NTZ in 2008. The 2014 dive survey also studied communities associated with two sites of similar maerl-gravel habitat inside and outside the NTZ, and detected no significant differences between the two.
A MSc research project (Allerton 2020) used photo quadrats from dive transects of benthic communities to compare sites inside the NTZ with those unprotected and exposed to scallop dredging. Species richness of benthic communities in the NTZ was nearly double that of the unprotected sites. For the majority of the organism groups their percentage cover was significantly greater (up to double) in the NTZ compared to the unprotected sites, e.g. macroalgae, Bryozoa, Porifera, Cnidaria. The study compared the biological traits of species (e.g. biomass, length, longevity, feeding mode, position they inhabit within the seabed) and found that the functional diversity of these traits were significantly higher in the NTZ. There was no difference though in functional evenness, and there was not a clear distinction in the trait composition amongst some unprotected and NTZ sample sites. This work indicates that whilst the same types of biological traits and functions are present in both the NTZ and the unprotected sites, there appears to be a greater variety and coverage of these in the NTZ which are important factors in determining the resilience of habitats, e.g. to climate change.
Summary
The results of monitoring studies undertaken in the NTZ are starting to provide evidence of positive changes (for aspects of species’ biology, such as scallop and lobster, and for seabed communities).
These studies, and interpretation of the results, remain subject to the difficulties/challenge of discerning changes arising from the management measures from natural variability.
This highlights the need for further research and monitoring over a longer time period to ascertain the full effects of the NTZ and MPA (see below). Nevertheless, it is worth noting that the cessation of fishing activity within the NTZ and MPA will have prevented further degradation and will have provided protection for sensitive and more vulnerable species and habitats, with the associated wider system benefits to ecosystem resilience and biodiversity (Boulcott et al., 2018).
It is also relevant that the management measures for the South Arran MPA include prohibition of scallop dredging across a much wider area, encompassing additional maerl, maerl gravel and seagrass habitats. A monitoring project has been set up to study the long-term effects of these measures and will in the future provide evidence of the effects of spatial fisheries management on biodiversity here.
In addition, research recently undertaken in the overlapping South Arran MPA have indicated that heterogeneous, shallow habitats such as gravel and maerl-based habitats and seagrass beds are important for gadoid species particularly juvenile cod (Elliott et al., 2016, Elliott et al., 2018). Therefore whilst the NTZ and overlapping South Arran MPA were not established for fish species, the protection of these habitats for juvenile fish could contribute to supporting their local recruitment and recovery.
Loch Carron MPA, Scotland
Background
Loch Carron MPA is situated on the west coast of Scotland in the Highland region, to the east of Skye. The MPA encompasses part of the sea loch and coastal waters to the south down towards Kyle of Lochalsh. It protects flame shell beds and numerous maerl beds (Figure 2).
Loch Carron was designated as an MPA on an urgent basis in May 2017 following damage to sensitive flame shell beds present in the outer part of the loch by a scallop dredger which was operating legally at the time. Alongside the urgent designation in 2017 a Marine Conservation Order was put in place that prohibited the use of towed, bottom-contacting fishing gear, i.e. trawling and dredging to protect the flame shell beds from further damage.
Subsequent survey work undertaken in 2017 recorded maerl beds and additional flame shell beds including one thought to be the largest in the world, located in the adjacent tide-swept Strome Narrows (Moore et al., 2018). Due to the scale and importance of these features, a public consultation on making the MPA and its associated management measures permanent was undertaken in 2018. The site was designated on a permanent basis in March 2019. A new Marine Conservation Order was also established that continued to prohibit towed, bottom-contacting fishing gear but extended the spatial protection across an extended site boundary and encompassing the maerl beds feature as well.
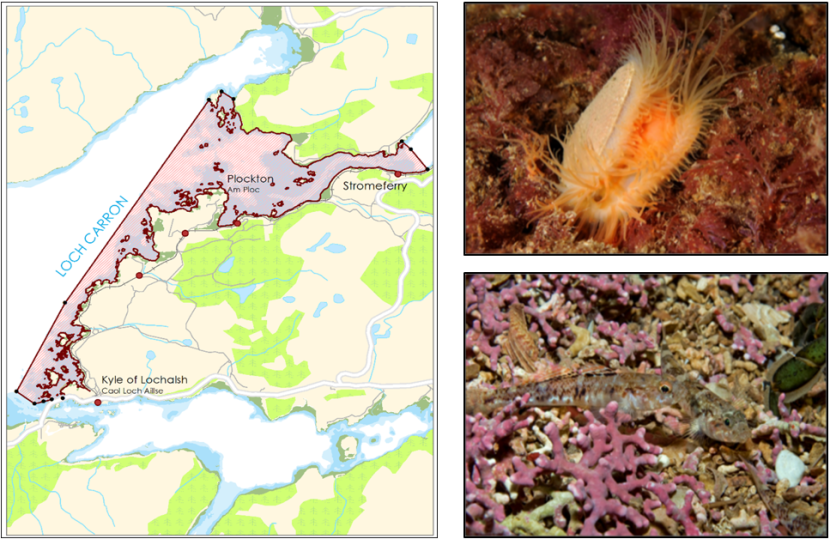
Three images are show. On the left the map shows the extent of the Loch Carron MPA spanning from the Kyle of Lochalsh in the south up to the coast north of Plockton and into Loch Carrron towards Stromeferry. To the right an image of an exposed flame shell with orange tentacles and below this gobies on a pink maerl bed.
Review of effectiveness
Loch Carron has only been designated recently with management put in place to remove/reduce fishing pressures on the seabed habitat features. Therefore this section provides an initial review of the condition of the affected flame shell beds and their potential for recovery in the future. The damage caused to the flame shell beds in Loch Carron was patchy with areas subject to disturbance and some habitat removal. Moore et al., (2018) provides a summary of the current condition of the features of the MPA. The damage from the dredger to Sgeir Bhuidhe flame shell bed largely took the form of flattening of the byssal turf and some disaggregation of the byssus/stone matrix. At the most intensively studied sites in 2017 it was found that significant numbers of Limaria had managed to persist within the impacted areas and at one site there was some evidence to suggest that the level of dredge scarring may have decreased due to byssal growth in the three month period between initial impact and the subsequent detailed diver studies. However, dredge scars were still visible in 2019, two years after the dredging occurred, at two sites on the north Sgeir Bhuidhe bed with distinct parallel dredge tracks apparent at one site (Moore, 2020). This area was close to the bed boundary where the flame shell habitat was relatively poorly developed. The physical habitat has been significantly modified here, although some recolonization of the parallel lines of pebbles and cobbles by Limaria hians may have taken place between 2017 and 2019 (Moore 2020). Diver sampling is required to determine the scale of any recolonization.
Given the presence of an extensive area of un-impacted flame shell habitat both immediately around Sgeir Bhuidhe and large, healthy beds elsewhere in Loch Carron and adjacent Loch Alsh, the potential for recolonisation through larval recruitment is considered high. On this basis, Moore et al., (2018) thought it likely that the rate of habitat recovery would be of the order of 10 years, considerably faster than that predicted in other studies, i.e. Trigg & Moore (2009). Whilst it is premature to comment on the effectiveness of management measures in the MPA in terms of leading to habitat recovery, the measures have thus far ensured that no further damage occurs and ongoing survey activities have confirmed the persistence of the protected seabed habitat features throughout the site.
Skomer Marine Nature Reserve and Marine Conservation Zone, Wales
Background
The Skomer Marine Conservation Zone (MCZ) was established in 2014 and is situated around the island of Skomer and the Marloes Peninsula in Pembrokeshire, south west Wales. Before 2014 the area had been Wales’ only Marine Nature Reserve for 24 years (established in 1990). Skomer MCZ has focal species and habitats including grey seal, pink seafan, sponge communities, seagrass and algal communities. The MCZ has an advisory committee which meets annually, made up of around 40 individuals and organisations with an interest in the area. In addition there is a research and monitoring programme to improve knowledge about the species and habitats in the MCZ which includes the production of an annual summary report and annual scientific reports.
Fishery byelaws prohibit the use of mobile fishing gear (dredges and beam trawls) and the taking of certain scallop species by any means within the MCZ. Skomer had feature status, conservation objectives and monitoring in place for its MNR features before it became a MCZ (including scallops, sponges, seagrass, etc). These still remain under transitional arrangements of the Marine and Coastal Access Act until the new MCZ protected features of the MCZ and their conservation objectives are decided and put in place.
Review of effectiveness
Due to the length of time these objectives and detailed monitoring programmes have been in place for the MNR, Skomer offers insights into the natural variability of habitats. The management measures put in place have enabled a diverse range of features to persist over a considerable period of time. The MCZ project status report 2016 (Lock et al., 2017) presented additional sediment infauna community survey for the MCZ, adding to the time series started in 1993 (when the site was a MNR). The last five surveys have shown the infauna community to be consistently healthy and species rich. Burton et al., (2018) provided the latest status report for specific features within the site. This included reports on the status of sponges, pink sea fan (Eunicella verrucosa) and seagrass beds. The sponge species present in the site continue to be very biodiverse (having one of the highest diversity of sponges in the UK) and the sponge community structure remains stable. They also reported an increase in the extent of the seagrass bed (particularly from 2010 in the east where habitat appears suitable for growth) from data collected through a variety of methods between 1982 and 2018. The seagrass bed area in 2018 was well above the minimum level specified for good condition in the MNR plan from 2000. However there had been a loss of pink sea fans between 1994 and 2018 (32 known losses). When examined against human activity in the vicinity of losses, lobster potting was the most frequently observed activity. Burton et al., (2018) note that there are no direct observations of damage, other unobserved activities could have caused the loss and additional data on activities would help to improve management.
Within the Skomer MCZ a scallop survey is conducted every four years. The 2016 results showed another increase in density of the king scallop (Pecten maximus) across the whole MCZ, with the 2016 survey estimating the density of scallops to be 35 scallops / 100 m2, which is an increase from the last survey in 2012, continuing the increasing trend seen in previous surveys.
Lyme Bay, England
Background
Lyme Bay, on the south west coast of the UK, supports a diverse assemblage of reefs, formed of mudstone, limestone, chalk and granite outcrops, cobbles and boulders, listed under Annex I of the Habitats Directive (Sheehan, 2013). The varied nature of the reefs support diverse marine communities. They are home to pink sea fans (Eunicella verrucosa), Ross (Pentapora fascialis), varied branching sponges and the commercially important king scallop (Pecten maximus) (Sheehan et al., 2013; Singer & Jones, 2018). It was the dredging for these scallops that caused the breaking up of mudstone reefs, overturning of boulders and removal of the slow-growing and distinctive pink sea fan and associated communities (Munro, 2012; Sheehan, 2013).
Concerns over the impact of demersal fishing and dredging in the area resulted in the voluntary closure of two areas within Lyme Bay, totalling in 2001. Two more areas were further protected through voluntary agreements in 2006, totalling 22 km2. However not all fishermen appeared to adhere to the voluntary closure and damage continued to be recorded (Munro, 2012). In 2008, 206 km2 of Lyme Bay were protected under a Statutory Instrument, creating an MPA, which closed the area to demersal fishing (including trawling and dredging) encompassing all known reef habitat (Sheehan et al., 2013). Static gear (pots and nets) and scallop diving were permitted.
A range of incentives were used, including legal, economic and public engagement which allowed extensive stakeholder input (Singer & Jones, 2018). This was particularly important as Lyme Bay was a commercially valuable area for fishermen, so legal deterrents, financial incentives and stakeholder participation were key in the successful designation and management of Lyme Bay MPA (Munro 2012, Singer & Jones, 2018).
Review of effectiveness
Lyme Bay MPA was assessed using the MPA governance framework analysis (Jones et al., 2013). Under the effectiveness element, which looks at whether management objectives are being fulfilled, based on the proportion and degree to which impacts are being addressed, Lyme Bay MPA is at level 3 (out of 5) - ‘some impacts completely addressed and some are partly addressed’ (Singer & Jones, 2018). There is legislation in place to restrict bottom-towed gear to support the recovery of the reef habitat. Research in the area provides a baseline for monitoring the impacts of bottom-towed gear on fisheries objectives.
A Defra funded study was undertaken over three years following the closure examining the seabed habitats and specific indicator species in areas in the new closure (NC) and comparing these against areas that had been previously unfished under voluntary conditions (CC) and areas which continued to be fished (Attrill et al., 2012). Results indicated that in the NC the community assemblage was starting to differ from what was seen in the fished sites, but there were also changes in the CC area as well, suggesting that both locations were in a state of change. The authors envisaged that in time the NC’s species assemblage would reach a more stable state and ‘catch-up’.Sheenan et al. (2013) showed that areas in the MPA considered to be mixed sediment between rocky reefs were colonised by reef-associated species, revealing that these areas were in fact sediment veneers on rock. The richness and abundance of these sessile reef fauna increased after the management was in place. Despite the impacts on communities from extreme storm events in 2013/14 (described in Sheehan et al. 2017) additional sampling effort demonstrated that communities were able to recover from this in subsequent years and the trajectory of recovery has been continuous (Davies et al. 2021, Davies et al. in prep).
Davies et al. (in prep) outlines results from studies of benthic and mobile species in the MPA over a period of 11 years since management was implemented compared to open control areas (OC) where fishing still occurs. Data collected from underwater towed video and baited cameras were used to examine diversity of taxa and functional traits (i.e. different feeding mechanisms, mobility and longevity of species). The number of taxa and functional trait richness were highest in the MPA and increased by 34.7% and 52.1% respectively, compared to OCs where these measures decreased by 9.7% and 20.1% respectively. Functional redundancy (the numbers of species that have the same traits) was greater in the MPA and increased over time, compared to the OC where it decreased. This can be indicative of areas of greater fishing pressure, where fewer species are present with unique traits that enable them to persist. Functional redundancy is important as the greater overlap in species improves resilience to pressures such as storms and disease. Filter feeders increased by 23% in the MPA compared to the OC, where swimming and crawling species increased. The authors outline that this is likely due bottom contacting gear reducing the presence of sessile filter feeds and increasing the abundance of mobile scavengers. They highlight that this shows the effectiveness of measures in protecting more sessile reef species, e.g. Ross (Pentapora fascialis), pink sea fan (Eunicella verrucosa). Overall they concluded that the data indicates a trend towards a more diverse and resilience rocky reef habitat in the MPA compared to the OCs.
Davies et al. (2021) describes how exploited and non-exploited fish and invertebrates responded to the management put in place inside the MPA compared to open control (OC) sites over the same 11 year period. Using baited remote underwater video systems they found that the number of taxa significantly increased inside the MPA compared to the open controls and abundance increased in both. For exploited fish species (including pollack (Pollachius pollachius), grey gurnard (Eutrigla gurnardus), red gunard (Chelidonichthys cuculus) and Chelidonichthys lucerna), red striped mullet (Mullus surmuletus), dab (Limanda limanda) and thornback ray (Raja clavata)) there was a 430% increase in the total number of taxa and 370% in the total abundance over the period. It is suggested that increases in habitat availability and improvements in reef complexity, e.g. increases in sessile reef fauna and flora, may have contributed to this. There was also an increase in the number of taxa in OCs but to a lesser degree, potentially showing some spillover effects. Non-exploited fish (including Gobiidae sp. Blenniidae sp., whiting (Merlangius merlangus)) did not show such changes and Davies et al. (2021) suggest this maybe due to competition for resources with the exploited fish species. No significant trends were found in exploited invertebrates within the MPA but they were found in greater abundance in the OCs. Potting for whelks, brown crab and lobster has continued in the MPA, which are three of the five species examined by Davies et al. (2021). Levels of potting increased in the MPA following the exclusion of towed bottom gear (Mangi et al. 2011) and Rees et al. (2021) suggest that this increase in effort has potentially removed a proportion of the increased abundance. Davies et al. (2021) suggest it would be useful to look at the abundance data alongside landings to help ascertain if there have been any benefits of the MPA for these species. For the non-exploited invertebrates studied (including common starfish (Asterias rubens), harbour crab (Liocarcinus depurator) and velvet swimmer crab (Necora puber)) there was a trend of decreased number of taxa and overall abundance in the MPA which they suggest maybe due to increased predation and competition. There was also a reduction in the abundance of non-exploited invertebrates in the OC which could be due to a displacement of species and changes in fishing effort.
Lyme Bay is considered a case study for such a closure and provides evidence for reef recovery. It was recognised by Attrill et al. (2012) that data from the initial monitoring 2 years after the fishing closure was not going to be long enough for most of the species of interest (indicator species) to re-establish and grow in areas which had previously been impacted by towed fishing gear. The long-term monitoring of the changes occurring on the reef communities has continued annually since 2008 and recent analysis (Davies et al. 2021, Davies et al. in prep) shows that recovery of these habitats is occurring. This analysis shows that the richness and abundance of taxa has significantly increased, highlighting the important role long-term monitoring plays in demonstrating the positive impact management has had. Building on the success of the yearly monitoring work to date, the study will enter its 14th year in 2021, further contributing to this unique long-term dataset.
Lundy Island, England
Background
Lundy Island is located in the Bristol Channel in the UK and was designated as a voluntary marine nature reserve in 1972, which later became a statutory Marine Nature Reserve in 1986 recognising the rich variety of marine life in the location (Irving, 2006). The area became a Special Area of Conservation (SAC) in 2005 for its reef, subtidal sandbanks, seacaves and grey seals. Lundy also designated as a Marine Conservation Zone (MCZ) in 2010 for spiny lobster (Palinurus elephas). In addition to the variety of habitats and species Lundy has other commercially important species such as lobsters, crab and scallops. A No Take Zone (NTZ) was established in 2003 and covers an area of approximately 4 km2 off Lundy’s east coast in which all fishing has been prohibited for the purpose of nature conservation. It was the first legally enforced no-fishing area in UK waters (Lundy Field Society). The rest of the area only allows crab and lobster potting. The aim was that the NTZ had a number of long-term benefits that included the following (Irving, 2006):
- increasing populations of fish and shellfish stocks within and outside the closed area,
- greater catches of fish around the edges of the closed area,
- increasing the wealth of marine life, and
- increasing benefits to local economies from tourism, diving and research.
Review of effectiveness
An annual monitoring programme to study the effects of the NTZ commenced in 2004 and ran till 2007 (Hoskin et al., 2009). It was focused on the effects of the NTZ on:
- commercial species (lobster, edible crab (Cancer pagurus), velvet swimmer crab (Necora puber), spider crab (Maja squinado),
- populations of scallops (Pecten maximus), and
- sessile species on rocky habitats particularly Eunicella verrucosa, axinellid sponges, ross coral (Pentapora foliacea) and dead man’s fingers (Alcyonium digitatum).
Data collected within the NTZ were compared against similar data from nearby control locations (within 1-5 km of the NTZ) and for lobsters and crabs there was a large scale comparison with reference locations that were several tens of km away.
Hoskin et al. (2011) compared the abundance and size of lobsters and crabs between the Lundy NTZ and two fished areas between 20-100 km away using data from 2004-2007. There was evidence of greater abundance and larger sizes of lobsters within the NTZ compared to the sites outside the zone where potting is allowed. In addition more lobsters were above the minimum landing size inside the NTZ. There was also evidence of spill-over of sub-legal sized lobsters from the NTZ to adjacent areas. The NTZ also appeared to cause a small, but significant increase in the size of brown crab (Cancer pagurus) and a decrease in the abundance of velvet crabs (Necora puber), the latter it is presumed due to predation and/or competition from lobsters. Scallops showed no changes in either abundance or size that indicated an effect of the NTZ between 2004-2007 (Hoskin et al., 2009). The scallops in both the NTZ and control locations were at very low densities and mostly comprise large, old individuals and there appeared to have been no significant recruitment in either location for several years.
In relation to the NTZ aspiration of ‘increasing wealth of marine life’ the monitoring reported but Hoskin et al. (2009) and later monitoring in 2014-15 (Vance and Ellis, 2016) note the natural variability in the sessile invertebrates between the NTZ and the control sites due to physical differences in the localities. Vance and Ellis (2016) confirmed the earlier conclusions of Hosking et al. (2009) that there appears to be no strong effects of the NTZ designation resulting in increased abundance of sessile marine invertebrates. However, the primary beneficiary of the NTZ designation was not intended to be the sessile marine invertebrates. In addition, the monitoring undertaken was not able to characterise what effect the NTZ may have on sessile invertebrate populations further afield from the monitoring sites.
These results indicated that the NTZ has been effective for some commercially important species but it is inconclusive for the wider biodiversity benefits that were aspired to. This may relate to the monitoring focus on sessile epifauna of rocky habitats and also because there was relatively low levels of fishing in NTZ prior to closure due to fisheries agreements in relation to the MNR. Therefore, monitoring was not examining looking at habitats where high intensity fishing pressure from demersal mobile gear e.g. dredging was removed.
Californian Marine Protected Areas
Background
In 2012 California completed its MPA network and it and now has 124 sites covering 16% of their state waters (852 square miles or 2207 km2) (Murray and Hee, 2019). The MPAs range in size and span the coastline from the Mexican border to the Oregon border, protecting a variety of marine habitats and species. Approximately half of the MPAs prohibit the take of any marine resources in fully protected State Marine Reserves (SMRs) and the other half allow some form of commercial, recreational, and/or tribal take in State Marine Conservation Areas (SMCAs).
California has made significant investment in MPA management, focusing on scientific monitoring, interagency coordination, awareness raising and enforcement. They established baseline monitoring in each of four distinct coastal regions over the period 2007-2018. A state-wide MPA monitoring framework was established to guide baseline monitoring efforts and provide the foundation for regional long-term monitoring plans. In 2018, they adopted a more targeted state-wide MPA Monitoring Program Action Plan that includes indicators, sites and species as well as objectives and methods to direct long-term, post-baseline monitoring. It also identifies opportunities to use MPA data to help inform management related to other California ocean priorities, including fisheries and climate change. In all, the state is investing $17 million in long-term MPA monitoring between 2018 and 2021 and the information gathered (Murray and Hee, 2019). There has also been a collaborative effort including the statutory agencies, native tribes, business, fishing sector, research and communities to undertake and enforce wide-ranging management measures. For example additional legislation to improve enforcement, citizen science surveys, mobile apps for fishermen on the location of MPAs, technologies to help identify poaching hotspots and fish tagging by anglers (Murray and Hee, 2019).
Review of effectiveness
Murray and Hee (2019) note that it may take years or even decades to accurately understand regional trends and measure changes that may be attributable to state MPAs. However, data from the baseline monitoring program and other sources, particularly data from older MPAs indicate that they are beginning to show signs of being effective. There has been an increase in commercially important fish species, e.g. lingcod and black rockfish in the central coast MPAs compared to similar fished habitats over a 5 year period. The endangered black abalone (Haliotis cracherodii) has increased in size inside the MPAs in this area and red abalone (Haliotis rufescens) populations in the North Central Coast's Sea Lion Cove SMCA, showed a sharp increase in the five years since it was established in 2010.
Monitoring in Californian MPAs with the longest history of protection shows that, over time, fish biomass of several economically important species targeted by fishermen continues to increase inside the MPAs at a greater rate than outside MPA boundaries, e.g. 2012 monitoring results from Point Lobos SMR, which was originally protected in 1973 and data from 2014 to 2016 at Point Cabrillo SMR, which was first protected in 1975. In the older MPAs of the Northern Channel Islands, targeted fish species had 1.5 times higher density and 1.8 times higher biomass inside reserve boundaries five years post-MPA implementation. In 2015, scientists found that biomass of targeted fish increased consistently inside all MPAs in the network. The average biomass for targeted fish species inside Northern Channel Islands reserves increased by 52% between 2008 and 2013 and increased 23% outside MPA boundaries during the same period. Additionally, abundance of three of the five targeted invertebrate species at the Northern Channel Islands, including California spiny lobster (Panulirus interruptus), warty sea cucumber (Parastichopus parvimensis), and red sea urchin is higher inside these same older MPAs.
Modelling and DNA work has confirmed significant spill-over of fish eggs into areas near to the MPAs and that these are a source of recruits for resident fish populations (Harada et al., 2015). Baetscher et al. (2019) found evidence of connectivity between populations of kelp rockfish in several Central Coast MPAs, and between populations in protected MPA areas and in fished populations.
The older Californian MPAs have also shown that positive effects extend beyond enhanced fisheries. A 2018 study of an MPA at the Northern Channel Islands that has been closed since 1978 found that competitive pressure from abundant, native algae in this older reserve likely reduces the success of the invasive alga, Sargassum horneri (Caselle et al., 2018).
Hauraki Gulf Marine Park, New Zealand
Background
The Hauraki Gulf Marine Park is located of the east coast of Auckland in New Zealand and was established in 2000. It covers an area of more than 1.2 million ha, including 50 islands and 5 marine reserves. The Act establishing the marine park set out objectives for the Gulf, its islands and catchments, and aims to achieve integrated management across land and sea. It also provided an integrated management approach to the Gulf, including the Resource Management Act, Conservation Act and Fisheries Act. To support this work, the Act also established the Hauraki Gulf Forum to bring together management by the local and central government, facilitate coordination, and recognise the special relationship the tangata whenua (Maori) have with the Gulf. Commercial and recreational fishing activity was allowed to continue throughout the park apart from a few small reserves (0.3% of the park). There has been a marked decline in aspects of the ecosystem and some fish stocks described below (Hauraki Gulf Forum, 2020) and in May 2019 the Hauraki Gulf Forum set two major goals; 1000 km2 of shellfish restoration and at least 20% marine protection of the Hauraki Gulf Marine Park.
Review of effectiveness
The Act requires the Hauraki Gulf Forum to produce a State of the Environment Report every three years, with the most recent one being published in 2020 (Hauraki Gulf Forum, 2020). Some fish species have been identified as needing efforts to rebuild their stocks with actions now being undertaken. There has also been a decline in the availability of harvestable cockles in the last 20 years where harvesting is allowed year round. There have been increases in monitored sites where seasonal harvesting bans are in place. The crayfish population has been substantially reduced and the species is considered functionally extinct in the most heavily fished areas and fishers are struggling to catch their quotas. Large reductions in catch allowances have been made to try to help the stocks recover.
Research presented in the State of the Gulf report suggests that the reductions in crayfish and tamure (snapper) have resulted in ecosystem impacts. Urchin populations have been allowed to grow without natural predation from these species, which has subsequently caused the loss of kelp forests and it is not clear whether the restrictions now in place for crayfish and tamure will be enough to reverse this decline. Additionally, the report suggests that the fishing related fatalities of Tāiko (black petrels) are unlikely to be sustainable. Fatalities have declined but there is still estimated to be a 70% likelihood that mortality rates from commercial fishing are greater than what the population of threatened Tāiko can sustain.
Spatial management measures for fisheries and other purposes
Isle of Man
Background
The Isle of Man has a series of closed or restricted areas within the 3 mile inshore limit, most of which are in place to provide protection or enhancement for the scallop fishery (e.g. Port Erin Closed Area and Ramsey Bay Fisheries Management Zone), or to act as trial reseeding or ranching areas (e.g. Laxey Bay and Niarbyl Bay Restricted Areas) (Figure 3). Surveys are focussed on assessing king and queen scallop stocks in the fisheries management zones using government enforcement vessels or commercial fishing vessels.
The most recent surveys have primarily been within the fisheries management zones of the Ramsey Bay Marine Nature Reserve (RBMNR). The fishing industry has direct involvement in the management of this site and scientific data helps the fishermen to make decisions about the appropriate levels of fishing or the extension of closures within the FMZ. In addition to the RBMNR three other areas (Douglas, Niarbyl & Laxey) were closed (2016-2017) to enable scientific monitoring including an assessment of the seabed habitat, scallop density, age and size distributions. Monitoring these protected areas will enable comparisons with unprotected areas to be made and the data can also provide information about how effective the closed areas are and how they respond to the absence or reduction of fishing activity.
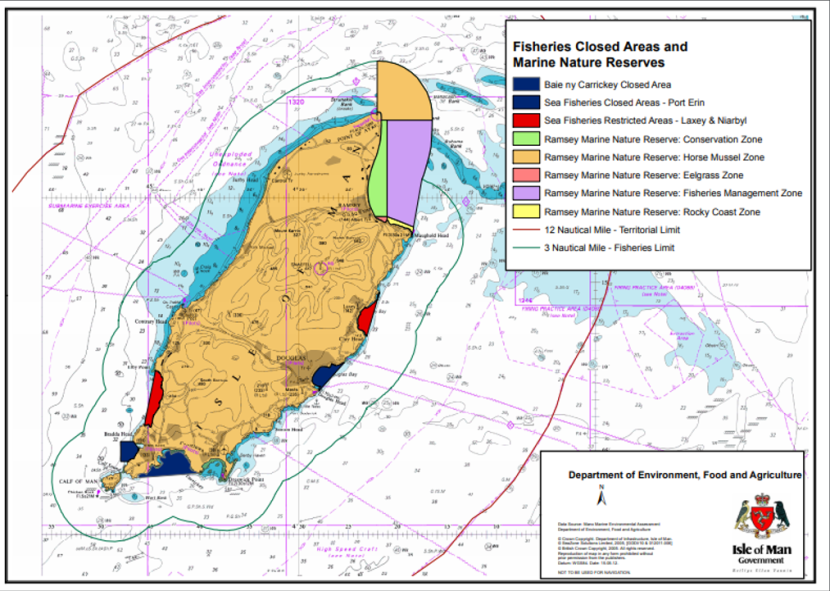
There are closed areas for fisheries on the east and west sides of the southern tip of the Isle of Man and on the east coast. There are marine reserves for horse mussels, seagrass and rocky coast as well as fisheries management zones in the north east of the isle.
Review of effectiveness
Previous work has established that Port Erin which has been closed to fishing since 1989, has higher densities of the king scallop (Pecten maximus) inside the closed area than outside (Beukers-Stewart et al., 2006). Reproductive output is also higher inside the closed area (Beukers-Stewart et al., 2005). The use of a particle tracking model has indicated that there is considerable connectivity around Isle of Man amongst the different known scallop grounds (Neil and Kaiser, 2008). This would suggest that a network of protected/restricted areas would increase the resilience of the scallop population from over-exploitation.
North East Coast UK Sandeel Closure
Background
The International Council for the Exploration of the Sea (ICES) recommends that local depletion of sandeel aggregations by fisheries should be prevented, particularly in areas where predators congregate. During the 1990s colonies of black-legged kittiwakes in eastern mainland Scotland that were adjacent to an area where high sandeel fishing pressure had recently developed, suffered from breeding failure. In 1999, the U.K called for a moratorium on sandeel fishing adjacent to seabird colonies along the U.K. coast and in response the EU requested advice from ICES. An ICES Study Group, was convened in 1999 and concluded that there were two reasons for continued concern about this area that provided the basis for a precautionary closure; sandeels in the area supported a number of potentially sensitive seabird colonies (Lloyd et al., 1991) and work on population structure indicated that sandeels in this region were reproductively isolated from the main fished aggregations in the North Sea (Wright et al., 1998), which has since been confirmed from further evidence (Wright et al., 2019). The EU advised that the fishery should be closed as a precaution whilst maintaining commercial monitoring. The fishery off the north-east UK coast was closed to commercial fishing in 2000, with the exception of a maximum of 10 boat days in each of May and June for stock monitoring purposes. Therefore this area serves both fisheries and biodiversity objectives. Following subsequent reviews for the EU by Marine Scotland Science (MSS) (Wright et al., 2002) and STECF (2007), this precautionary closure has been maintained and following the exit from the European Union in 2021 the closure has been retained under UK legislation.
Review of effectiveness
Marine Scotland Science (MSS) surveys of the area found an initial increase in sandeel abundance during the period of the closure (Greenstreet et al., 2006) due to a relatively large recruitment in the first year of the closure, which would not have been related to any recovery in the spawning stock. MSS dredge surveys also indicated a detectable decrease on total mortality on age 1+ sandeels following the closure and there have been some large year-classes produced in the area which have contributed to higher abundance and stock recovery (Wright et al., 2002; Regnier et al., 2017). Kittiwake breeding success appeared to have benefitted from the reduction in mortality on age 1+ sandeels (Daunt et al., 2008). For colonies on the mainland coast (Scotland and England) the breeding success of kittiwakes has tended to be higher since the fishery closure than in the preceding five years, although the local stock dynamics are mainly driven by recruitment rather than changes in fishing mortality (MCCIP, 2018). Much of the inter-annual variation in sandeel recruitment in the area can be explained from thermal relationships affecting both the development of sandeels and their copepod prey (Wright et al., 2019; Régnier et al., 2019).
STECF (2007) reviewed the closure in relation to goals and objectives proposed by the ICES study group and found that the closure was effective with respect to these. Mitchell et al., (2018) also concluded that the results of kittiwake breeding success suggest that the sandeel fishing closure in eastern Scotland and north-east England has been effective. The closure is considered to be one of the ‘other area based measures’ that contribute to the MPA network in Scotland because it protects sandeels, a MPA network feature (Cunningham et al., 2011).
Skagerrak coast, Norway
Background
The Skagerrak is a strait of water running between the south-east coast of Norway, the west coast of Sweden and the Jutland peninsula of Denmark. It connects the North Sea and Kattegat Sea area which leads to the Baltic Sea. In September 2006, three marine protected areas were established in the Norwegian area of the Skagerrak to protect fish and shellfish stocks, primarily for lobster. These areas are the Bolærne MPA in the outer Oslofjord (0.7 km2), the Flødevigen MPA in Arendal (1 km2) and the Kvernskjær MPA in Hvaler (0.5 km2) (Thorbjørnsen et al., 2018). Capture of lobster has been effectively banned in the MPAs since 2006 through fishing gear restrictions, and for fish only hook and line fishing is allowed in the protected areas (Pettersen et al., 2009).
Review of effectiveness
A before-and-after (BACI) annual study from 2006 to 2010 (Moland et al., 2013) found that during the four years after being designated all three of the MPAs studied had greater increases in the number and size of European lobster (Homarus gammarus) compared to fully fished areas. Before designation, lobster abundance (as catch/unit effort) was typically similar in all areas. Over time, abundance increased at all sites (inside and outside MPAs) but increased more in the MPAs, and after four years had increased by 245% in MPAs and by 87% in fully fished areas. Before designation, lobster size was similar across areas but over time, size increased at all sites, but more in the protected areas (12-15% in MPAs and by 3% in fully fished areas). Thorbjørnsen et al., (2018) found that lobsters moving from MPAs and caught in fished areas were significantly larger than lobsters moving out of control areas (unprotected areas). In instances where lobsters tagged in a control area moved into an MPA, the immigrating lobsters had a larger body size than the mean in their area of origin. The range of movement undertaken by recovered lobsters extended beyond the home range sizes suggested by previous shorter-term studies.
Moland et al. (2013) also examined the effects of the Flødevigen MPA on Atlantic cod (Gadus morhua). The partial protection of Atlantic cod in the MPA was followed by an increase in population density and body size compared with control areas further away. Prior to protection, there was no clear difference in the expected proportion of traps containing cod amongst the MPA and the control sites. After designation (2007–2010) the MPA consistently had the highest expected proportion of traps with cod. Post designation of the MPA the MPA had the largest 90 per cent percentile length and by 2010, the large-size component in the MPA was 8 cm longer than in any of the control regions, and 17 cm longer than cod from the nearest control region surrounding the reserve. By 2010, cod in the MPA were on average 5 cm longer than in the control (unprotected) areas. Moland et al. (2013) note that their results support to the notion that MPAs may help to counter evolutionary impacts of harvesting on cod through restoration of size–structure. However, they highlight that the positive effects documented could be attributed to increased survival of individuals in the MPA displaying extreme site fidelity, a behaviour that might not be representative for the population norm.
Inner Sound & BUTEC range, Scotland
Background
The Inner Sound is a body of water located on the west coast of Scotland, separating the Inner Hebridean islands of Skye, Raasay and South Rona from the Applecross Peninsula of the Scottish mainland. It includes some of the deepest areas in Scotland’s territorial waters reaching over 300 m in places. The seabed is mainly comprised of muddy sediments with steeply sloping rock faces along the coast. There are fisheries restrictions in this area that include the Southern Inner Sound Protected Area and the British Underwater Test and Evaluation Centre (BUTEC) range. The BUTEC range is an underwater military test and evaluation range used by the Ministry of Defence and Royal Navy for noise ranging of surface ships and submarines and for testing of a variety of weapons and sensors. Within the Southern Inner Sound Protected Area there is a seasonal closure on certain fishing gears (dredge, beam trawl, demersal trawl or demersal seine net) as outlined in the Inshore Fishing (Prohibition of Fishing and Fishing Methods) (Scotland) Order 2015. There has been a closure preventing all types of fishing in the BUTEC range since 1975 but the shape of the range has changed over time (Maclay et al., 2008, Military of Defence byelaws Highland). The closures have been put in place because of the MOD use in these areas and there are no fishery or biodiversity objectives for the closures.
Review of effectiveness
As there are no fisheries or biodiversity objectives to the Inner Sound closures there isn’t any specific monitoring set up to determine changes resulting from the closures. However, there is some survey information available on the types of habitat present and associated species. Areas that have been surveyed in the Inner Sound (a deep channel to the west of the Crowlin Islands) have noted extensive coverage of high quality ‘burrowed mud’ habitat which is a Priority Marine Feature (PMF) in Scottish waters (Moore, 2000; Moore & Atkinson, 2012). The mud supports a dense megafaunal burrowing community and good numbers of tall seapens Funiculina quadrangularis and fireworks anemones Pachycerianthus multiplicatus. Adey et al., (2012) noted significantly fewer F. quadrangularis in trawled areas compared to non-trawled areas in the BUTEC range. The northern feather star Leptometra celtica (another PMF) is also widely distributed here and forms dense aggregations on mixed substrates. Flame shell beds and deep maerl bed habitats have been recorded in a number of locations recently in the outer BUTEC area and are likely to be more widespread (Moore et al., 2018; Shucksmith et al., 2021). These features as well as the aforementioned species are sensitive to abrasion caused by mobile demersal fishing gear (outlined in FeAST) and their presence and distribution is likely to be reflective of the fisheries management measures in place within the BUTEC area. Additionally, a survey of Nephrops in the BUTEC range (summarised in McLay et al., 2008) observed very large animals, consistent with fishermen’s knowledge and practice of deploying creels at the boundaries of the inner management zone to try to attract larger animals. It is thought that the range may act as a refuge for large Nephrops.
Case studies of approaches for reviewing the effectiveness of measures to manage fisheries in MPAs in Scotland
Studies have been set up in locations where management measures are anticipated to cause the biggest changes to the environment, at the following locations with the following target habitats: Sound of Barra (maerl beds), South Arran (maerl beds and burrowed mud), Small Isles (burrowed mud), Wester Ross (flame shell beds, maerl beds), Loch Sunart to the Sound of Jura (mixed sediment habitats) and Lochs Duich, Long and Alsh (mixed sediment habitats).
Studies are designed around the collection of video and grab data from within replicated areas of similar physical characteristics (“monitoring boxes”) both inside and outside a management zone. Slightly different approaches are taken depending on the target habitat and species.
Figure 4 illustrates this design on the Sound of Barra, where boxes were located inside and outside of the MPA boundaries in anticipation that those outside would not receive management and act as controls to those inside.
Over the next few years, and by the time of the next MPA Report to Parliament in 2024 (as required under the Marine (Scotland) Act 2010), we expect to be able to reflect on changes in the abundance of faster-growing, sensitive epifaunal species (e.g. ascidians, large anemones and hydroids) and changes in the composition of infaunal communities associated with habitat. This may help to indicate that a system as a whole is responding to a reduction in disturbance, even if changes amongst the slow growing target habitats and species are not yet detectable.
Research contracts to investigate which elements of the ecosystems lend themselves to being used as indicators in this way are currently being prepared and results will inform the design of monitoring studies over the next three years to feed into the 2024 MPA Report to Parliament.
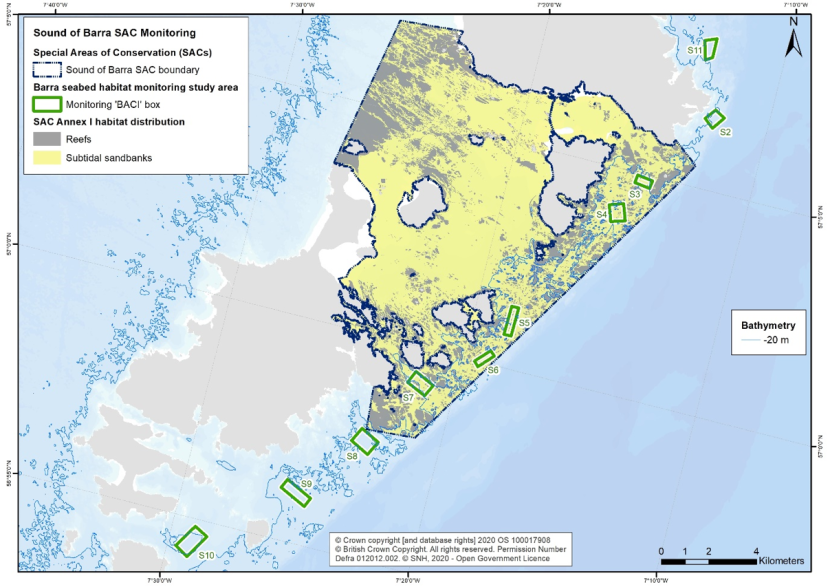
There are 10 monitoring boxes along the eastern coastline spanning from Vatersay, Barra to South Uist. Five of these are within the Sound of Barra SAC boundary.
Discussion
As noted in the introduction, this paper did not aim to provide a comprehensive analysis of the effectiveness of fisheries management measures in MPAs but the key issues identified through these are discussed under five discrete headings below.
Our existing knowledge justifies the need for management - we just need to allow adequate timescales for detecting and evaluating change.
We have a good and growing evidence base for MPA features relating to their sensitivity to fishing pressures (FeAST). This has led to the development of management advice in MPAs for a reduction or removal of fishing pressure to reduce the risk of fishing related impacts and enable the achievement of conservation objectives.
Many of the current case studies available are from relatively young MPAs and management restrictions. The result is that some have not reported any change in habitat condition or where evidence is presented for benefits/change this is in relation to the size and abundance of shorter lived features with higher recoverability rates e.g. scallops, crab. Additionally, MPAs including those in Scotland, were selected based on habitats and species being in good condition in locations where human activities that cause deterioration are absent or low intensity. Consequently, these would not be the areas where we would expect to see the greatest change. However, just because change/improvement in the protected features of an MPA is not detected over a short timescale does not mean that the management isn’t effective. Management will be helping to reduce pressures that we understand have been or could damage protected features and will reduce the risk of future damage and deterioration. Case studies from older MPAs such as the increasing infaunal species richness in Skomer MCZ and the diverse algal community resilient to invasive species in Californian MPAs, illustrate it can take decades to see positive changes in some habitats and species. Conversely, there are examples where fisheries management has not been in place or has been ineffective where we have seen impacts on both habitats, e.g. Loch Carron, and ecosystem scale effects, e.g. the Hauraki Gulf kelp ecosystem. It may be that where changes have not been detected that not enough time has passed, what is being monitored is unsuitable for picking up the changes that are happening or that possibly the management is insufficient and additional measures may be needed to achieve the conservation objectives.
It is challenging to set up robust frameworks to monitor and detect change in the marine environment, especially in the context of climate change.
As outlined at the start of this paper, there are numerous challenges to designing monitoring and assessment processes to detect, interpret and report on the effectiveness of management in the marine environment. However, the most pressing and complex is trying to do this against the background of the continuous changes that marine ecosystems go through and the overlying addition of pressures associated with climate change, e.g. sea level rise, ocean acidification, de-oxygenation. Alongside this there is often difficulty in identifying suitable controls for assessing managed areas against, i.e. areas with similar habitats and environmental conditions that can be maintained in the long-term because environmental conditions, activities and management in these areas can change. Also the condition of the features at the time the management is established is an important consideration - where the habitat is in better condition there is less likely to be as much change detected as in an area in poorer condition. This can lead to issues in the interpretation of the results that are achieved from studies and so framing of the evidence in light of the management and MPA objectives, and communication of the results are exceptionally important (see below).
Further development of a useful, relevant evidence base framed around conservation objectives is required.
There is a far greater body of evidence on this topic from tropical marine environments. Due to the differences in ecosystems, their species composition and associated growth rates these aren’t readily applicable to Scotland’s temperate marine environment and so weren’t included in this review. There is a need for further robust work to be undertaken (addressing the issues raised in the points below) and published in relation to temperate marine ecosystems. There is likely to be additional published and grey literature pertaining to this subject that has not been gathered and reviewed as part of this paper. There would be benefit in undertaking a more systematic review to be used as the starting point that could be built on as evidence from MPAs in Scotland and other temperate countries develops.
Baseline reference locations are needed to help inform our understanding of how the condition of marine habitats and species are changing over time. The establishment and monitoring of these areas alongside locations with fisheries management restrictions will help improve the evidence-base. Examples of monitoring for MPAs in Scotland (outlined in section 5) that look at the effects of fisheries management compared to control areas outside of management will help to meet this need.
For those temperate studies reviewed here, there was a lot of variation and, in some cases a lack of clarity, over the objectives relating to a particular MPA or fisheries management measure. Without the objectives being clear and upfront it is difficult to evaluate the effectiveness of the management measures. Any further work we or others do, to expand this initial review should carefully consider the objectives of the MPA and/or fisheries restrictions in evaluations of their effectiveness. Whilst consideration should be given to the conservation objectives of the protected features of the MPAs, this shouldn’t preclude consideration of evaluations on the effects of management on indicator species/habitats in the wider ecosystem. Not only can this approach help us examine effects of management over shorter timescales but it can offer opportunities for discussions around the wider ecosystem benefits of fisheries management with stakeholders (see below).
Communication on the effectiveness of fisheries management in MPAs needs to be relevant to the conservation objectives and stakeholders - the attributes monitored are key in doing this.
Whilst obviously we need to be able to demonstrate the effectiveness of change for protected features of MPAs, the case studies outlined have demonstrated that there are species of fish and shellfish which may respond positively (in terms of size and abundance) in shorter timescales to fisheries management measures, e.g. scallops, crabs and lobster. Selecting short-term indicator species as part of a wider monitoring programme for MPAs would allow us to demonstrate that management measures are being effective for improving biodiversity and have wider species benefits. Therefore, consideration should be given to selecting an appropriate range of both protected habitat/species attributes and indicator species in monitoring established for MPAs. Species such as lobster, juvenile gadoids and scallops utilise many of the more sensitive habitats the MPAs in Scotland were set up to protect, e.g. reefs, seagrass beds and maerl; particularly in their juvenile stages. Being able to convey how fisheries management for protected habitats has wider benefits for such species of commercial interest would also likely prove a valuable stakeholder communication tool and help ensure compliance with MPA-related fisheries management measures.
Monitoring programme design should reflect the likely timescales of change and be representative of the locations and features in MPAs so that the results can be used to support assessments for the MPA network as a whole.
There is a need to invest time and resources to detect change as a result of management measures, especially where the species and/or habitat is slow growing and long-lived. The longer-term monitoring plans of the Scottish MPA Monitoring Strategy are a commitment to doing this. Creating an evidence base of direct causal relationships between management measures and positive ecosystem effects is a huge undertaking of cost and time and would be prohibitive to undertake for every MPA in Scotland. This is recognised by the Scottish MPA Monitoring Strategy which sets out principles for prioritising monitoring effort. For some MPAs, assessment of what is likely to happen to the protected features in the long-term will therefore need to be based on evidence from the scientific community and others, experience from elsewhere and the results from comparable case studies. A representative subset of locations, features and attributes that cover, in particular those MPAs where recover conservation objectives have been set and measures are expected to result in the most obvious change are likely to form a core focus for monitoring. The results will help enable us to better understand the likely impact of management measures in other MPAs. It is important though that monitoring programmes also include a health check of sites in good condition at the time of designation (those with ‘conserve/maintain’ conservation objectives) to provide wider context (including in relation to natural variability) and ensure that significant changes in features can be identified.
Conclusion
Through this review of case studies of relevance to Scotland there is evidence that:
- The abundance and size of shellfish and crustaceans including scallops, crab, lobsters and crayfish responds positively when spatial fisheries closures are in place, with effects being seen within 5 -10 years. The evidence is strongest for lobster but this is perhaps due to it travelling relatively less compared to crab species.
- Commercial fish species increase in biomass and abundance within areas where relevant fishing activity is restricted, usually within around 5 - 10 years, with spill-over effects outside of these areas. This effective is likely to be more notable for more residentiary species.
- The condition (extent, diversity) of habitats and their associated sessile species e.g. soft sediments, reefs, kelp beds, seagrass remains at least stable or increases where spatial fisheries management is implemented, and is most evident over longer time scales (decades).
- Improvements in condition are most evident where MPA features are in deteriorated but recoverable state, but for other MPA features spatial fisheries management reduces risk of damage and increases resilience to future change.
- The time since fisheries measures were implemented within MPAs in Scotland (five years) is generally not enough to be able to detect change in protected features (e.g. due to slow growth rates, and/or sporadic recruitment), although initial recovery may be seen relatively quickly where conditions allow, e.g. Loch Carron where a specific event was known to cause damage.
- The spatial management of fishing activities in MPAs is proven method for conserving protected features in temperate marine environments, including Scotland.
Being able to assess the efficacy of management measures in MPAs is key to supporting future adaptive management of the MPA network in Scotland.
References
Adey, J.M., Atkinson, R.J.A., Smith, I.P., Tuck, I.D. & Taylor, A.C. 2012. The Environmental Impact of the Nephrops Creel Fishery. SNH Commissioned Report ROAME No. F02-AA405.
Allerton, A.J. 2020. Investigating the effects of a highly protected marine area on ecosystem functioning of a temperate benthic community. MSc dissertation, Department of Environment and Geography, University of York. Y3874665.
Attrill, M.J., Austen, M.C., Bayley, D.T.I., Carr, H.L., Downey, K., Fowell, S.C., Gall, S.C., Hattam, C., Holland, L., Jackson, E.L., Langmead, O., Mangi, S., Marshall, C., Munro, C., Rees, S., Rodwell, L., Sheehan, E.V., Stevens, J., Stevens, T.F. & Strong, S. 2011. Lyme Bay - a case-study: measuring recovery of benthic species; assessing potential “spillover” effects and socio-economic changes, 2 years after the closure. Response of the benthos to the zoned exclusion of bottom towed fishing gear and the associated socio-economic effects in Lyme Bay. Final Report 1. June 2011. Report to the Department of Environment, Food and Rural Affairs from the University of Plymouth-led consortium. Plymouth: University of Plymouth Enterprise Ltd. 108 pages.
Baetscher, D. et al., 2019. Dispersal of a nearshore marine fish connects marine reserves and adjacent fished areas along an open coast. Molecular Ecology, 1-13.
Beukers-Stewart, B.D., Vause, B.J., Mosley, M.W.J. & Brand, A.R. 2006. Closed areas and stock enhancement of scallops - What's the catch? Journal of Shellfish Research, 25: 267-268.
Beukers-Stewart, B.D., Vause, B.J., Mosley, M.W.J., Rossetti, H.L. & Brand, A.R. 2005. Benefits of closed area protection for a population of scallops. Marine Ecology Progress Series 298:189-204.
Boulcott, P., Stirling, D., Clarke, J. & Wright, P.J. 2018. Estimating fishery effects in a marine protected area: Lamlash Bay. Aquatic Conservation: Marine and Freshwater Ecosystems. 28: 840-849.
Burton, M., Lock, K., Newman, P., Jones, J. 2018. Skomer Marine Conservation Zone Project Status Report 2018. Natural Resources Wales Evidence Report 324.
Caselle, J.E. et al., 2018. Marine management affects the invasion success of a non-native species in a temperate reef system in California, USA, Ecology Letters, 21: 43-53.
Chaniotis, P, Robson, L.M., Lemasson, A.J., Cornthwaite, A.L., Bower, R. & Howell, K.L. 2019. UK deep‐sea conservation: Progress, lessons learned, and actions for the future. Aquatic Conservation: Marine and Freshwater Ecosystems, 1-19.
Crimmins, E. 2018. The Influence of the Lamlash Bay no-take zone, Firth of Clyde, on spatial and Temporal Variation in the Recovery of Commercially Exploited Crustaceans. MSc Thesis North Yorkshire: University of York, 43.
Cunningham, S., Gillham, K., Chaniotis, P.D., Crawford-Avis, O., Linwood, M. & Payne, O. 2011. Assessing the contribution of other area-based measures to the ecological coherence of the MPA network in Scotland’s seas. Report produced by Scottish Natural Heritage, the Joint Nature Conservation Committee and Marine Scotland for the Scottish Marine Protected Areas Project.
Daunt, F., Wanless, S., Greenstreet, S. P. R., Jensen, H., Hamer, K. C., and Harris, M. P. 2008. The impact of the sandeel fishery closure on seabird food consumption, distribution, and productivity in the northwestern North Sea. Canadian Journal of Fisheries and Aquatic Sciences, 65, 362-381.
Davies, B.F.R, Holmes L., Rees, A.,Attrill, M.J., Cartwright, A.Y. & Sheehan, E.V. 2021. Ecosystem Approach to Fisheries Management Works - how switching from mobile to static fishing gear improves populations of fished and non-fished species inside a Marine Protected Area. Journal of Applied Ecology.
Davies, B.F.R, Holmes L., Bicknell, A., Attrill, M.J., & Sheehan, E.V. In preparation. A Decade Implementing Ecosystem Approach to Fisheries Management Improves Diversity and Ecosystem Function Within a Marine Protected Area in the UK. Diversity and Distributions.
Douarin, M., Elliot, M. Noble, S.R., Sinclair, D. Henry, L., Long, D., Moreton,S. G., & Roberts, J.M. 2013. Growth of north-east Atlantic cold-water coral reefs and mounds during the Holocene: A high resolution U-series and 14C chronology. Earth and Planetary Science Letters, 375, 176-187.
Dyman, J.M. 2022. The Lamlash Bay No-Take-Zone: a case study for the importance of community-led marine protected areas for biodiversity conservation and sustainable fisheries in Scotland and around the world. MSc dissertation, Environmental Department, University of York.
Elliott, S.A.M., Ahti, P.A., Heath, M.R., Turrell, W.R. & Bailey, D.M. 2016. An assessment of juvenile Atlantic cod distribution and growth using diver operated stereo-video surveys. Journal of Fish Biology.
Elliott, S.A.M., Allan, B.A., Turrell, W.R., Heath, M.R. & Bailey, D.M. 2018. Survival of the fittest: explanations for gadoid imbalance in heavily fished seas. Aquatic Conservation: Marine and Freshwater Ecosystems, 28(5), pp. 1192-1199.
Greenstreet, S.P.R, Armstrong, E., Mosegaard, H., Jensen, H., Gibb, I.M., Fraser, H.M., Scott, B.E., Holland, G.J., & Sharples, J. 2006. Variation in the abundance of sandeels Ammodytes marinus off southeast Scotland: an evaluation of area-closure fisheries management and stock abundance assessment methods. ICES Journal of Marine Science, 63(8), 1530-1550.
Hall-Spencer, J.M. & Moore, P.G. 2000. Scallop dredging has profound, long-term impacts on maerl habitats. ICES Journal of Marine Science, 57, 1407-1415.
Hauraki Gulf Forum. 2020. State of our Gulf 2020.
Hepper, B. T., & Gough, C. J. 1978. Fecundity and rate of embryonic development of the lobster, Homarus gammarus (L), off the coast of North Wales. Journal du Conseil Permanent International pour l’Exploration de la Mer, 38, 54-57.
Hiddink, J.G., Jennings, S., Sciberras, M., Szostek, C.L., Hughes, K.M., Ellis, N., Rijnsdorp, A.D., McConnaughey, R.A., Mazor, T., Hilborn, R., Collie, J.S., Pitcher, C.R., Amoroso, R.O., Parma, A.M., Suuronen, P. & Kaiser, M.J. Global analysis of depletion and recovery of seabed biota after bottom trawling disturbance. Proceedings of the National Academy of Sciences, 114(31), 8301-8306
Hold, N., Beaumont, A.R. & Kaiser, M.J. 2010. Progress Report: Genetic investigation into the spill over of scallop larvae from Port Erin closed area. Fisheries & Conservation Report No. 18, Bangor University. pp10.
Hoskin, M.G., Coleman, R.A., & Von Carlshausen, L. 2009. Ecological effects of the Lundy No-take Zone: the first five years (2003-2007). Final report to Natural England, DEFRA and WWF-UK.
Hoskin M.G., Coleman R.A., Von Carlshausen, L. & Davis, C.M. 2011 Variable population responses by large decapod crustaceans to the establishment of a temperate marine no-take zone. Canadian Journal of Fisheries and Aquatic Sciences, 68, 185-200.
Howarth, L.M., Dubois, P., Gratton, P., Judge, M., Christie, B., Waggitt, J.J., Hawkins, J.P., Roberts, C.M. & Stewart, B.D. 2016. Trade-offs in marine protection: multi-species interactions within a community-led temperate marine reserve. ICES Journal of Marine Science, 74, 263-276.
Howarth, L. M., Roberts, C.M., Hawkins, J.P., Steadman, D.J., & Beukers-Stewart, B.D. 2015a. Effects of ecosystem protection on scallop populations within a community-led temperate marine reserve. Marine Biology, 162, 823-840.
Howarth, L.M., Pickup, S.E., Evans, L.E., Cross, T.J., Hawkins, J.P., Roberts, C.M. & Stewart, B.D. 2015b. Sessile and mobile components of a benthic ecosystem display mixed trends within a temperate marine reserve. Marine Environmental Research, 107, 8-23.
Huijbers, C.M., Connolly, R.M., Pitt, K.A., Schoeman, D.S., Schlacher, T.A., Burfeind, D.D., Steele, C., Olds, A.D., Maxwell, P.S., Babcock, R.C. & Rissik D. 2015. Conservation benefits of marine reserves are undiminished near coastal rivers and cities. Conservation Letters, 8, 312-319.
Huvenne, V.A.I., Bett, B.J., Masson, D.G., Le Bas, T.P. & Wheeler, A.J. 2016. Effectiveness of a deep-sea cold-water coral Marine Protected Area, following eight years of fisheries closure Biological Conservation, 200, 60-69.
IPBES. 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. S. Díaz, J. Settele, E. S. Brondízio E.S., H. T. Ngo, M. Guèze, J. Agard, A. Arneth, P. Balvanera, K. A. Brauman, S. H. M. Butchart, K. M. A. Chan, L. A. Garibaldi, K. Ichii, J. Liu, S. M. Subramanian, G. F. Midgley, P. Miloslavich, Z. Molnár, D. Obura, A. Pfaff, S. Polasky, A. Purvis, J. Razzaque, B. Reyers, R. Roy Chowdhury, Y. J. Shin, I. J. Visseren-Hamakers, K. J. Willis, and C. N. Zayas (eds.). IPBES secretariat, Bonn, Germany. 56 pages.
Irving, R.A. 2006. Lundy’s marine life – a balancing act of protecting and promoting. Proceedings of the 60th Anniversary Symposium of the Lundy Field Society, Devon, UK.
James, L. 2019. The recovery of the commercially valuable scallop species, Pecten maximus, Under Different Forms of Protection around the Isle of Arran. MSc Thesis, University of York, North Yorkshire, 54.
Jones, P.J.S., De Santo, E.M., Qiu, W. & Vestergaard, O. 2013. Introduction: an empirical framework for deconstructing the realities of governing marine protected areas. Marine Policy, 41, 1-4. doi:10.1016/j.marpol.2012.12.025.
Kaiser, M.J., Clarke, K.R., Hinz, H., Austen, M.C., Somerfield, P.J. & Karakassis, I. 2006. Global analysis of response and recovery of benthic biota to fishing. Marine Ecology Progress Series, 311, 1-14.
Kaiser, M.J., Hormbrey, S., Booth, J.R., Hinz, H., Hiddink, J.G. 2018. Recovery linked to life history of sessile epifauna following exclusion of towed mobile fishing gear. Journal of Applied Ecology, 55, 1060-1070.
Langton, R., Stirling, D.A., Boulcott, P. and Wright, P.J. 2020. Are MPAs effective in removing fishing pressure from benthic habitats and species? Biological Conservation.
Lock, K., Burton, M., Newman, P. and Jones, J. 2017. Skomer MCZ Project Status Report 2016. Natural Resources Wales Evidence Report 197.
Mangi, S. C., Rodwell, L. D., and C. Hattam, C. 2011. Assessing the Impacts of Establishing MPAs on Fishermen and Fish Merchants: The Case of Lyme Bay, UK. Ambio 40(5), 457-468.
Marine Scotland. 2017. Scottish Marine Protected Areas Monitoring Strategy.
McLay, H.A., Drewery, J.D., Dobby, H., Weetman, A. & Campbell, N. 2008. An assessment of the effects of the creel and trawl fishing zones on Nephrops stocks in the Loch Torridon area. Fisheries Research Services Internal Report No 16/08
Mercer, T., Kamphausen, L., Moore, J., Bunker, F., Archer Thompson, J. & Howson, C. 2018. South Arran MPA diver survey of maerl beds, kelp and seaweed communities on sublittoral sediment, and seagrass beds 2014. Scottish Natural Heritage Research Report No. 882.
Mitchell, I., Cook, A., Douse, A., Foster, S., Kershaw, M., McCulloch, N., Murphy, M. & Hawkridge, J. 2018. Kittiwake breeding success. UK Marine Online Assessment Tool.
Millar, H., O’Hara Murray, R., Gallego, A., Gormley, K. & Kent, F. 2019. Connectivity of selected Priority Marine Features within and outwith the Scottish MPA network. Scottish Natural Heritage Research Report No. 1048.
Moland, E., Olsen, E.M., Knutsen, H., Garrigou, P., Espeland, S.H., Kleiven, A.R., André, C. & Knutsen J.A. 2013. Lobster and cod benefit from small-scale northern marine protected areas: inference from an empirical before-after control-impact study. Proceedings of the Royal Society B: Biological Sciences, 280, 1754.
Moore, C.G. & Atkinson, R.J.A. 2012. Biological analyses of underwater video from research cruises in the Clyde Sea, Loch Torridon and the Inner Sound, the North Minch, Loch Eriboll and off Orkney. Scottish Natural Heritage Commissioned Report No. 536.
Moore, C.G., Harries, D.B., James, B., Cook, R.L., Saunders, G.R, Tulbure, K.W., Harbour, R.P. & Kamphausen, L. 2018. The distribution and condition of flame shell beds and other Priority Marine Features in Loch Carron Marine Protected Area and adjacent waters. Scottish Natural Heritage Research Report No. 1038.
Munro, C.D. & Baldock, B.M. 2012. Lyme Bay Closed Area: measuring recovery of benthic species in cobble reef habitats - analysis of data collected by SCUBA divers September 2008, August 2009 and July 2010. A Marine Bio-images report. Marine Bio-images, Exeter, Devon, UK.
Murray, S. & Hee, T.T. 2019. A rising tide: California’s ongoing commitment to monitoring, managing and enforcing its marine protected areas. Ocean & Coastal Management, 182.
Neill, S.P. & Kaiser, M.J. 2008. Sources and sinks of scallops (Pecten maximus) in the waters of the Isle of Man as predicted from particle tracking models. Fisheries & Conservation Report No. 3, Bangor University. pp. 25.
Pettersen, A.R., Moland, E., Olsen, E.M. & Knutsen, J.A. 2009. Lobster reserves in coastal Skagerrak: an integrated analysis of the implementation process. In Integrated Coastal Zone Management (eds Dahl E, Moksness E, Støttrup J.), pp. 178-188 London, UK: Wiley-Blackwell Publishing.
Pilkey, O. & Pilkey-Jarvis, L. 2007. Useless arithmetic - Why environmental scientists can’t predict the future. Columbia University Press, pp 230.
Rees, S. E., Mangi, S. C., Hattam, C., Gall, S.C., Rodwell, L.D., Peckett, F.J., and Attrill, M. J. 2015. The socio-economic effects of a Marine Protected Area on the ecosystem service of leisure and recreation. Marine Policy 62,144-152.
Rees, S. E., Ashley, M., Evans, L., Mangi, S., Sheehan, E.V., Mullier, T., Rees, A. & Attrill, M.J. 2021. An evaluation of the social and economic impact of a Marine Protected Area on commercial fisheries. Fisheries Research, 235.
Régnier, T., Gibb, F. M., and Wright, P. J. 2019. Understanding temperature effects on recruitment in the context of trophic mismatch. Scientific Reports, 9, 15179.
Rijnsdorp, A.D., Stefan, S.G., Garcia, C., Hiddink, J., Hintzen, N., van Denderen, D., & van Kooten, T. 2018. Estimating sensitivity of seabed habitats to disturbance by bottom trawling based on the longevity of benthic fauna. Ecological Applications, 28(5), 1302-1312.
Ross, R.E., Nimmo-Smith, W.A.M. & Howell, K.L. 2017. Towards ‘ecological coherence’: assessing larval dispersal within a network of existing Marine Protected Areas. Deep Sea Research Part I: Oceanographic Research Papers, 126, 128-138.
Sciberras, M., Jenkins, S.R., Kaiser, M.J., Hawkins, S.J. & Pullin, A.S. 2018. Evaluating the biological effectiveness of fully and partially protected marine areas. Environmental Evidence, 2, 4.
Sheehan, E.V., Stevens, T.F., Gall, S.C., Cousens, S.L. & Attrill, M.J. 2013. Recovery of a Temperate Reef Assemblage in a Marine Protected Area following the Exclusion of Towed Demersal Fishing. PLoS ONE, 8(12): e83883.
Sheehan, E.V., Cousens, S.L., Nancollas, S.J., Strauss, C., Royle, J., Attrill, M.J., 2013. Drawing lines at the sand: evidence for functional vs visual reef boundaries in temperate marine protected areas. Marine Pollution Bulletin, 76(1), 194-202.
Sheehan, E.V., Cartwright, A.Y., Game, C., Cousens, S.L., Bridger, D.R., Nancollas, S.J., Rees, A.G., Gall, S.C., & Attrill, M.J., 2017. Lyme Bay – a case-study: Response of the benthos to the zoned exclusion of towed demersal fishing gear in Lyme Bay; 8 years after the closure, March 2017. Report to Natural England from Plymouth University Marine Institute.
Shucksmith, R.J., Shelmerdine, R.L. & Shucksmith, R. 2021. Biological analyses of seabed imagery from within and around Marine Protected Areas in Orkney, Shetland, Inner Sound, and Islay and Jura in 2019. Scottish Marine and Freshwater Science, 12 (2).
Singer, R. & Jones, P. 2018. Lyme Bay marine protected area: A governance analysis. Marine Policy.
Stewart, B.D., Howarth, L.M., Wood, H., Whiteside, K., Carney, W., Crimmins, É., O’Leary, B.C., Hawkins, J.P. & Roberts, C.M. 2020. Marine Conservation Begins at Home: How a Local Community and Protection of a Small Bay Sent Waves of Change Around the UK and Beyond. Front. Marine Science, 7,76.
STECF (Scientific, Technical and Economic Committee for Fisheries) 2007. 26th Plenary Meeting report of the Scientific, Technical and Economic Committee for Fisheres (PLEN-07-03), 5-9 November 2007. ISPRA.
Thorbjørnsen, S.H., Moland, E., Brockstedt, M., Huserbråten, O., Knutsen, J.A., Knutsen, H. & Olsen, E.M. 2018. Replicated marine protected areas (MPAs) support movement of larger, but not more, European lobsters to neighbouring fished areas. Marine Ecology Progress Series, 595, 123-133.
Trigg, C. & Moore, C.G. 2009. Recovery of the biogenic nest habitat of Limaria hians (Mollusca: Limacea) following anthropogenic disturbance. Estuarine, Coastal and Shelf Science, 82, 351-356.
Vance, T. & Ellis, R. 2016. Lundy SAC: Subtidal reef condition assessment and No Take Zone benthic monitoring survey 2014/15. Final report. RP02178 Natural England.
Wright, P. J., Christensen, A., Régnier, T., Rindorf, A., Van Deurs, M. 2019. Integrating the scale of population processes into fisheries management, as illustrated in the sandeel, Ammodytes marinus. ICES Journal of Marine Science, 76, 1453-1463.
Wright, P.J., Jensen, H., Mosegaard, H., Dalskov, J. and Wanless, S. 2002 European Commission’s annual report on the impact of the Northeast sandeel fishery closure and status report on the monitoring fishery in 2000 and 2001. Report to EC DG XIV. FRS Marine Laboratory, Aberdeen, UK.
Appendix 1
|
Case study |
Background information |
Key conclusions |
|---|---|---|
|
Darwin Mounds SAC, Scotland |
|
|
|
Lamlash Bay No Take Zone, Scotland |
|
|
|
Loch Carron MPA, Scotland |
|
|
|
Skomer MNR and MCZ, Wales |
|
|
|
Lyme Bay, England |
|
|
|
Lundy Island, England |
|
|
|
Californian MPAs |
|
|
|
Kauraki Gulf Marine Park, New Zealand |
|
|
|
Isle of Man scallop closures |
|
|
|
North east coast UK sandeel closure |
|
|
|
Skagerrak coast, Norway |
|
|
|
Inner Sound & BUTEC Range, Scotland |
|
|
Disclaimer: Scottish Natural Heritage (SNH) has changed its name to NatureScot as of the 24th August 2020.
At the time of publishing, this document may still refer to Scottish Natural Heritage (SNH) and include the original branding. It may also contain broken links to the old domain.
If you have any issues accessing this document please contact us via our feedback form.






