Ecological Networks Protected Areas Review - Ecological Networks Think Piece
Ecological Networks - Connecting Scotland’s nature at the landscape scale

Photo credits: NatureScot
Authors
Alessandro Gimona1, Peter Wright2, Antonia Eastwood1. Alejandro Gallego2, Alison Hester1
1 The James Hutton Institute, Aberdeen, UK
2 MarineScotland Science, Aberdeen UK
Origin and purpose
The Board of NatureScot has recognised that there are significant challenges in protecting biodiversity, a major one being the fragmentation of landscapes and habitats, which limits the ability of species and habitat to thrive, and their resilience to environmental change. The Protected Areas Committee of the Board, in their effort to enhance the role of Protected Areas in nature conservation, has commissioned three think pieces on (i) Approaches for improving condition and biodiversity;(ii) Lessons that can be learnt from behavioural sciences to improve public engagement with protected areas; and (iii) the role of protected areas as a catalyst for resilient Ecological Networks. This think piece addresses the last topic, and is intended to summarise the principal issues, to propose some options for action, and to promote discussion during a webinar (and beyond).
Executive Summary
- The concept of ecological networks (ENs), has developed over the past 40 years with the broad aim of maintaining the integrity of ecological processes over landscapes(seascapes), and this is rooted in ecological understanding that was developed over the 20th century. The term “ecological network” was adopted by the IUCN and the Convention on Biodiversity (CBD) Conferences of the Parties.
Critical components
- Key components of an Ecological network are Core Areas, Buffer Zones and Ecological Corridors. In the marine environment core areas very often coincide with Marine Protected Areas.
- A resilient ecological network is one in which species can persist even in the face of natural perturbations and human activities; This is realised by supporting large-scale distribution and dynamics of species and communities.
- Large enough core areas in good habitat condition and functional corridors that allow movements of individual and genes are necessary conditions.
The human component is also crucial. Connectivity and resilience of PAs and their networks are not just biophysical entities on maps; they are the outcomes of the interactions between governance structures, different actors and users, the natural resources and the overall system.
- Diagnosing and assessing which variables across both ecological and social domains affect human behaviour, and therefore ecological outcomes, is critical for resilient, sustainable ecological networks.
Opportunities to provide a step change in habitat connectivity and resilience
- The level of protection afforded to biodiversity by Scottish Protected Areas is unclear. A gap analysis to understand whether biodiversity is adequately protected by the existing set of Protected Areas is desirable, and further research is also needed on ecological requirements of priority species to enhance the functioning of networks.
- Size, condition, and ecological coherence are all important factors of success. It is unclear if in Scotland there are functional networks of protected areas vs a portfolio of areas
- The existing set of Marine Protected areas (MPAs) could benefit from targeted spatial restrictions on fishing effort and, in some cases, by having shorter distances among protected aggregations.
- For ecological networks to be resilient and sustainable there needs to be alignment between the scale of ecological processes and of governance. Knowledge brokers or bridging actors have an important role in linking scales and actors that would be otherwise disconnected.
Integrating the management of PAs with the wider land/marine management
- Ecological networks are embedded in landscapes that have multiple land uses, and are affected by multiple drivers. To enhance biodiversity protection large enough portions of the landscape/seascape would need to be restored, or and/or harbour compatible economic activities.
- On land, Ecological Networks integrated with the well targeted restoration of peatlands, native woodlands and expansion of ‘High Nature Value” (HNV) farmland from existing ‘core’ example areas could provide multiple benefits and help with biodiversity conservation. Both a bottom-up and a top-down approach are needed, with a national-scale spatial strategy providing a framework for local initiatives and for the targeting on incentives. The nascent Regional Land Use Partnerships could lead the coordination of such an effort.
- Marine management has tended to maintain a distinction between fishery and nature conservation measures. However, this distinction is not consistent with international treaties that have expounded the ecosystem approach. Integrated ecosystem assessments used in marine management advice would be desirable. Targeting site protection measures in locations where both species of nature conservation concern and habitat of importance to commercial fish and shellfish occur might both help promote ecological networks and greater acceptance by affected stakeholders.
- Integration with the wider environment could be improved by re-orienting and targeting incentives, promoting public participation in network planning, and by matching governance and planning to the scale of the biophysical processes that we want to protect. Flexibility and adaptability of institutions and management practices would add to the ability to deal with environmental and societal change.
- Ecological networks could enhance resilience to climate change by allowing some species to remain in a favourable habitat. Well functioning networks would also be likely to allow new species, e.g. from the south of the UK or from continental Europe to keep up with changes in their climatic requirements. However, Ecological networks are not a panacea for climate resilience. Translocations might need to be considered in some cases.
Ecological Networks - Connecting Scotland’s nature at the landscape scale
Nature conservation thinking, since its birth, has been in evolution, influenced by cultural and scientific developments probably in equal measures.
Our understanding has been transformed in the last decades. Protected areas, while still the cornerstone of any conservation policy, have come to be seen as just an element of a wider set of instruments, while the social aspect of conservation has grown in importance.
The change in perspective in the last decades has been dramatic, with a shift from goals to protect local individual features to a much more systemic approach, spurred, also, by a better understanding of earth system science, landscape ecology, and social sciences.
1. Scientific origin of Ecological Networks
The term “ecological network” became established in Europe in the early 1990s and has been adopted by influential organisations such as IUCN and the Convention on Biodiversity (CBD) Conferences of the Parties.
The concept of ecological networks (ENs), however, has developed over the past 40 years with the broad aim of maintaining the integrity of ecological processes over landscapes, and this is rooted in ecological understanding that was developed over the 20th century.
The ecological insights on which ENs are based derive from the convergence of various scientific advancements. One of the principal ones is Island Biogeography (McArthur and Wilson, 1967) which, together with patch dynamics (Levins, 1969) laid the foundations for metapopulation theory later developed by Gilpin and Hanski (1991; Hanski and Gilpin, 1997).
Parallel to these developments was the evolution of landscape ecology, initially firmly rooted in the planning tradition of central and Eastern Europe. This was well established already in the 1960s (e.g. Mizerovsky, 1963; Hackett, 1964) and recognised the concept of cultural landscapes (e.g. Naveh, 1982) and filtered later into the english speaking literature (e.g. Allen et al., 1998). Landscape ecology had a renaissance in the 1980s in the USA which resulted in a new emphasis in anglo-american ecological circles on recognising that landscape pattern emerges from interactions between human and natural processes and that “ecosystem management” must include the human dimension (e.g. Kaufman et al,1994 ). Such interaction influences ecological flows in landscape mosaics, such as movements of water, nutrients, plant propagules, animals, and other materials.
A very important insight that followed from the synthesis of the European and American landscape ecological schools of thought was that people and Nature cannot be treated as two separate systems. This accelerated the process of re-integration of the human dimension in conservation. Nature conservation can only take place in dynamic landscapes that respond to multiple land use pressures, and, ultimately, are a socio-ecological system. These pressures often result in habitat fragmentation, which increases the vulnerability to extinction of a species by reducing the area of habitat available to local populations and by limiting opportunities for dispersal, migration and genetic exchange, thus creating metapopulations.
The concepts of metapopulation and landscape ecology have increasingly been applied also to marine systems (Kritzer and Sale 2006; Hidalgo et al., 2016; Pittman, 2018), although the dominance of hydrodynamic and to a lesser extent seabed processes in shaping population dynamics means that marine ecosystems tend to be more prone to natural change than those in terrestrial systems. While habitat fragmentation and patch mosaics may be less relevant to most highly mobile marine species, some long lived sedentary species now appear to be constrained to a few small habitat fragments (Stirling et al. 2016).
One of the products of putting the new understanding into practice were the first plans of ecological networks as an attempt to integrate biodiversity conservation into broad environmental management plans.
Because of their heterogeneous implementation in different countries, ecological-networks have been defined with different terminology. This includes “territorial system of ecological stability”, “reserve network”, “bioregional planning”, “ecoregion-based conservation”, “connectivity conservation areas”. However, there are commonalities behind the apparent heterogeneity. The approaches that are usually classified as ecological networks share at least two generic goals, namely: (i) to maintain the functioning of ecosystems to foster the conservation of species and habitats; and: (ii) to promote sustainable land use in order to reduce the impacts on biodiversity and/or to increase the biodiversity value of managed landscapes (Bennett and Wit, 2001).
Question 1
‘What are the critical components of an effective ecological network and what part can Protected Areas play in their development/strengthening?’
- Key components
In achieving these goals, a number of elements can be discerned which together characterize most ecological networks. These are: (i) the identification of critical areas that are in need of protection; (ii) a focus on conserving biodiversity at the landscape/seascape, or regional scale; (iii) an emphasis on maintaining or strengthening ecological coherence, by providing opportunities for connectivity; (iv) a stress on the need to buffer critical areas from the effects of potentially damaging external activities.
Ecological networks, therefore - at least in theory - comprise a system of ecologically important areas that are functionally connected to counteract the effects of landscape fragmentation.
Lawton et al. (2010) (reproduced in Figure 1), redrew a classic pictorial representation provided by IUCN.
2.1 Core Areas
Core areas are defined areas of landscape that are either legally protected (PAs) or managed by their owners as though they were (see also Box 1). In the marine environment, some core areas coincide with the Marine Protected Areas (MPAs). Ecologists tend to use the term in a functional sense, i.e. areas that are important to promote population viability. Their size and condition are of fundamental importance (see also section 3 below).
Significant opportunities often exist to integrate non protected sites into ecological networks. This is partly because the process of protection has been influenced by history and opportunity as much as scientific considerations.
2.2 Buffer zones
Buffer zones are transitional areas characterized by land uses compatible with nature conservation. In these areas, sited around the core areas, some land use restrictions are applied to protect them from potential damage. The spirit is one of reconciliation of biodiversity conservation and economic activities, as long as they are compatible with the protection of the core area. Which activities qualify is rather undefined and it depends, case by case on both biological considerations and social negotiations (see also Jongman and Troumbis, 1995; Sahfer, 2015).
2.3 Ecological Corridors
Ecological Corridors serve to maintain crucial ecological processes of exchange of individuals and genes between core areas, so they should be large enough to permit movement, also from a behavioural point of view. Movements connected with dispersal, migration foraging and/or reproduction can benefit from corridors.
Physical connection, despite being used as a proxy, is not necessarily enough to define a corridor. There are several species-specific considerations that make the same area a corridor for some groups of species and a barrier, or at least an area that is not crossed, for several other species In particular habitat-interior species that are subject to edge effects tend to avoid narrow corridors or areas disturbed by human presence. To better understand whether a linear feature is actually an ecological corridor, It is useful to review the in-depth debate that took place in the Netherlands about 20 years ago regarding effectiveness of corridors (e.g. van der Windt and Swaart, 2007), which produced guidelines for different types of species.(Broekmeyer and Steingrover, 2001). Guidelines have also been produced recently by IUCN.
Having said that, ecological corridors can be multifunctional and can also provide recreation and landscape amenity compatibly with the life history and tolerance to human disturbance of the species whose movements the corridor is aiming to promote. Clear objectives for a corridor are therefore important in order to prevent disappointment about what the corridor can achieve.
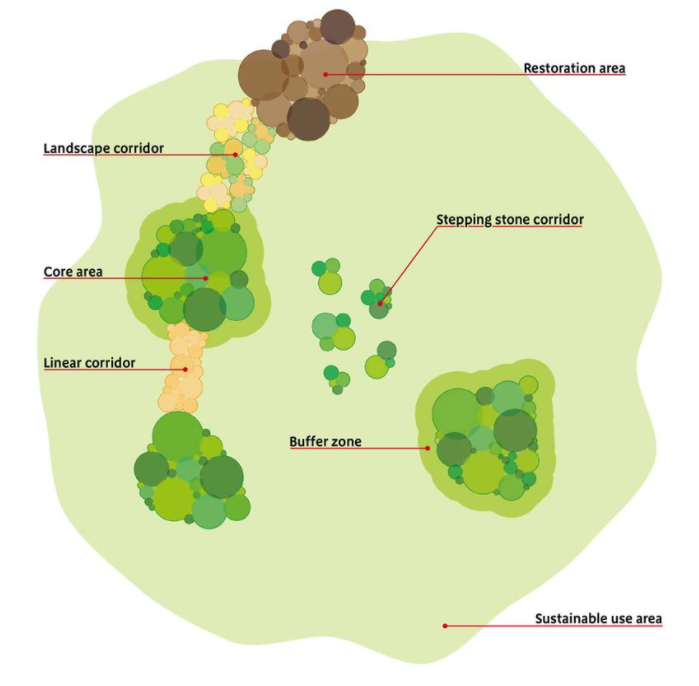
The image shows a simplified landscape with discrete core areas, important for biodiversity, that are isolated from one another.
It shows various ways in which the health of these core areas, and so their positive contribution to biodiversity, can be increased. This can be achieved through two main means; improvement of the broader landscape within which they sit so it is more hospitable towards biodiversity and also, through strategic connections between these core areas for biodiversity. These come in two main forms, stepping stones of habitat that are important for biodiversity and allow the movement between sites or, corridors between the core areas that directly connect them. Corridors can take the form of linear corridors of similar habitat to those areas they wish to connect or, landscape corridors that are broader areas with a mosaic of different biodiverse habitats.
These strategic corridors or stepping stones form the backbone of Nature Networks.
2.4 Marine Protected Area Networks
As in the terrestrial case, the effectiveness of a marine ecological network can be characterised by how well it supports the large-scale distribution and dynamics of species and communities. A resilient ecological network is one in which species can persist even in the face of natural perturbations and human activities (including climate change). Functional connectivity among habitat patches through migration, dispersal and gene flow is important in promoting populations that are resilient to external pressures. While continuous areas of protected habitat, like the terrestrial corridors, are not necessarily relevant in the sea, the distance that passively drifting organisms are transported by sea circulation or the active movements of mobile species are important to consider.
The lack of effective protection of marine ecological networks is evident from the many marine species whose distributional extent has declined, with some now extirpated from several parts of their historic range. Species most at risk tend to be long-lived, late maturing and vulnerable to capture or damage by fishing activities or other anthropogenic pressures. Due to the vulnerability of certain species and habitats there has been international support for the designation of Marine Protected Areas (MPAs), where some or all of the human pressures are stopped. As with Lawton’s (2010) review of Protected Areas, MPAs do have the potential to support ecological networks if sufficient, in ecological terms, core areas of suitable habitat for species and communities at risk are protected and where there is sufficient replication to support functional connectivity among habitat patches, so that populations are resilient to external pressures. While dependent on the scale of population processes and on the MPA size, the aggregations of rare species within individual MPAs are generally unlikely to allow self-sustaining local populations and so networks of Protected Areas that encompass a species’ range are important. When detrimental activities within MPAs are restricted, the size of the area for such measures accounts for both the activity and a buffer area to minimise any unintended straying close to the feature being protected.
While physical fragmentation is generally less obvious in marine than in terrestrial environments, fishing can lead to unsustainable levels of mortality for both the species caught and those damaged in the process, in addition to potentially altering the physical nature of the substrate (in the case of bottom-contact practices). This aspect is shared by other less-widespread human activities such as aggregate extraction or undersea mining. Some once widely distributed species are now largely restricted to areas where fishing does not operate or is much reduced. This has led to fragmented distributions despite there being many areas of suitable physical habitat remaining (Shepherd et al., 2012; Stirling et al., 2016; Langton et al., 2020). Habitat fragmentation is particularly detrimental to species whose connectivity is dependent on a passive dispersing life-stage, as self-recruitment and colonisation potential declines as habitat patches decrease in size and the distance among them increases.
The concepts of metapopulation and landscape ecology are being increasingly applied to consider how best to promote population growth and resilience in impacted marine species and communities (Kritzer and Sale 2006; Hidalgo et al., 2016; Pittman, 2017). As the risk of extirpation is generally higher in isolated aggregations with low exchange, information on patch size and connectivity alone can provide a useful guide to the effectiveness of networks (Hanski, 2004). However, knowledge of the interplay between connectivity (dispersal) and local population dynamics allows for a better understanding of persistence in a network. Using such an approach, Hastings and Botsford (2006) demonstrated that connectivity can be just as important as the productivity of a habitat patch in ensuring population persistence. While there are many applications of spatial population models to the development of MPA networks in coastal and tropical reef systems (e.g. Botsford et al., 2014), they were not used in the development of the UK and Scottish MPA networks.
The concept of source-sink dynamics, where birth rates are higher than death rates in the source habitat, is important to MPA design as it is important to protect areas that generate a surplus of offspring that are exported. As in the landscape sense, areas that act as a source are typically characterised by high quality habitats but in the marine realm the location relative to current flow and the persistency/variability of circulation patterns is also relevant because it affects the potential for larval export to other areas (e.g. Millar et al., 2019). Targeting and protecting source areas through MPA designation could have a major role in strengthening marine ecological networks because of the potential to supply adjacent unprotected areas (Kritzer and Sale 2006; Pittman, 2017). In most cases too little is currently known about the scale of connectivity and local productivity of areas to consider source-sink dynamics in the MPA designation process. However, there are examples where such information has informed the Scottish MPA network e.g. for sandeels (Proctor et al., 1998; Gibb et al. 2017).
Box 1- Key Definitions
|
Core area A defined area that is either protected by law or a OECMs (see below). Protected area A defined portion of landscape, legally protected, devoted to and managed for biodiversity conservation and (often) the conservation of cultural values. OECM (Other Effective Area-Based Conservation Measure) An area that is not legally protected but managed (e.g. by NGOs) in such a way to promote biodiversity conservation (IUCN WCPA, 2019). Functional connectivity The degree of unobstructed movement of individuals genes and propagules in the landscape between populations and ecosystems. Structural connectivity A measure of physical proximity based on the arrangements of habitat patches, elements. Structural connectivity is often presumed to be a proxy for functional connectivity but the latter also depends on a species’ life history. Ecological corridor A portion of landscape devoted to facilitating functional connectivity and often managed and protected to ensure long-term viability Metapopulation A population composed of groups of spatially separated populations that exchange individuals or genes. Ecological network A system of core areas connected by ecological corridors, created to conserve biological diversity, particularly in fragmented landscapes.
|
In summary, Protected Areas, when connected by dispersal, are often able to function as core areas of an ecological network, and can be crucial for the survival of the populations of conservation concern by providing breeding and foraging habitat. Many of the protected areas in Scotland are likely to have this potential. However, as will be discussed in the next question, both size and condition are important for PAs to qualify as core areas.
2.5 Humans as critical ‘components’ of ecological networks
Facilitating, maintaining and managing the critical components of ecological networks cannot be divorced from the social-ecological systems that they are an integral part of. This can be from the monitoring of conservation features; the expectations of visitors; formal and informal policies and rules (institutions, in the sense of rules-in-use) that determine how land is governed and managed; local livelihoods that are dependent on them or the ecosystem services that flow from them (Cumming & Allen, 2017). Therefore the ecological integrity of ecological networks and their management should not be viewed as sole problems of ecology, for ecological scientists alone to solve. Protected Areas (PAs) are not ecological islands, but are embedded in and function as social-ecological systems, at different nested scales, from individual sites to sea/landscapes. Social-ecological systems are complex and evolving. Facilitating effective management of ecological networks requires an understanding and diagnosis of the causal relationships between critical components and variables of the social-ecological system that interact together to affect ecological network performance (connectivity, resilience etc). Diagnosing and assessing which variables across both ecological and social domains affect human behaviour, and therefore ecological outcomes, is critical for resilient, sustainable ecological networks (Ostrom et al., 2007). These variables, however, may be very different in different systems and contexts. For example, the governance systems and institutions managing and regulating common pool resources such as fish harvesting are very different to those in, for example, a farmer who owns the land that an SSSI includes. A commonly used social-ecological framework (Figure 2 below) to help understand and diagnose socio-ecological systems has been developed and built upon by Ostrom and colleagues (McGinnis & Ostrom, 2014).
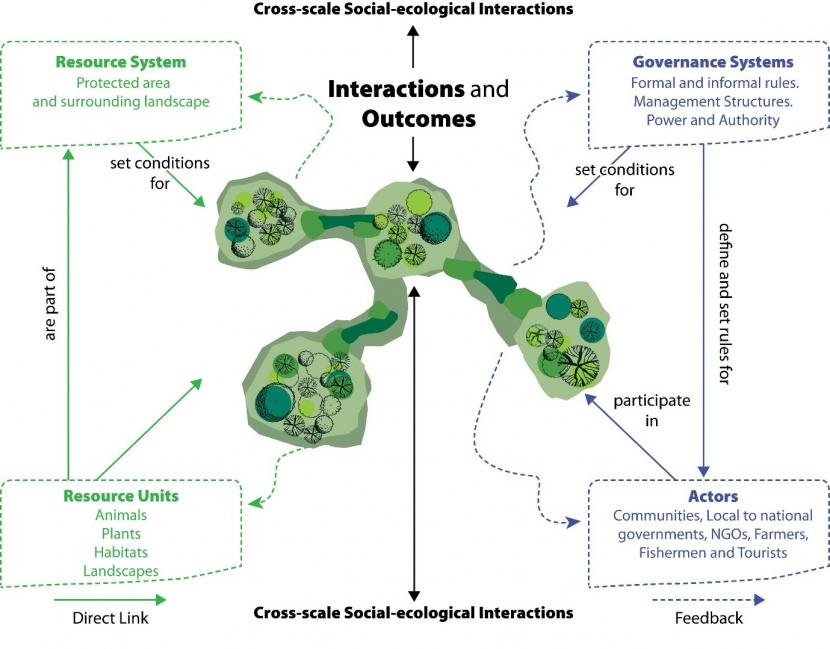
A commonly used socio-ecological system framework (adapted from McGinnis & Ostrom, 2014). Resource System and Resource Units comprise the ecological side of the framework. Governance Systems and Actors comprise the social side of the framework. The two sides of the framework interact to produce the ecological network outcomes (centre). The focal social-ecological system can be considered as a logical whole but is also subject to exogenous influences from related ecological systems or social-economic-political settings.
Summary-Q1
Key ecological components of an Ecological Network are Core Areas, Buffer Zone and Ecological Corridors. The latter are less defined in the marine environment. Many Scottish PAs can function as core areas.
A resilient ecological network supports large-scale distribution and dynamics of species and communities. Effective ecological networks maintain the functioning of ecosystems and enable species and communities to persist even in the face of natural perturbations and human activities. Protecting core areas can foster the conservation of species and habitats and reduce the impacts on biodiversity but the effectiveness of such measures will depend on how well these support core/source areas and ensure connectivity.
The human component, often neglected, is also a critical part of an Ecological Network.
People (actors), who construct the formal and informal rules, norms and polices on how land/sea is managed are an integral and critical component part of any Ecological Network. PAs could have a stronger role to play by having a better understanding on the casual links between the governance systems of PAs, human behaviour and its impact on species and habitats in an ecological network.
Question 2
What opportunities exist for our Protected Areas to provide catalysts to promote a step change in habitat connectivity and resilience?
There are significant opportunities to build resilient wide-scale ecological networks using as a starting point the existing set of Protected Areas, both on land and in the sea. However to succeed it would be necessary to deepen our scientific understanding of the present situation.
- Systematic gap analysis
The purpose of such an analysis is to take stock of whether biodiversity is adequately protected by the existing set of PAs. Recent monitoring results are not encouraging for several species groups, and this leads to doubt that all species of conservation interest are adequately protected.
There is evidence that relatively small and informal networks of habitats such as between some woodlands and grasslands, often promoted by forward-thinking local authorities are in place in various areas of Scotland. Part of the Central Scotland Green Network is likely also to qualify as an ecological network in the technical sense described above (depending on the functional attributes of their core areas and corridors). These are very good starting points. However, since the ecological rationale ecological networks is to meet the objective of halting and reversing biodiversity loss, wide coverage and explicit protection is crucial. Because the existing set of Protected Areas was not designed from the beginning as part of an ecological network, or of a comprehensive portfolio, it is likely that gaps in protection exist, and it would be desirable to fill them.
A starting point to provide a catalysis to expand connectivity is a portfolio of complementary core areas that include, as far as possible, irreplaceable elements (i.e. species and habitat types that are not found anywhere else), and representative occurrences of Scotland’s semi-natural habitats and native species (e.g. Margules and Pressey, 2000).
Gaps could be assessed, for example, by analysing the proportion of threatened bryophytes, vascular plants, and of BAP priority species and habitats falling into PAs. Identified gaps would guide the selection of complementary core areas which would need protection.
The level of assessment should be nested, and therefore carried out at the level of the individual core area; at the level of a portfolio of core areas; and at the level of a network of core areas.
Redundancy, namely representation by multiple examples, provides insurance against local destruction of important habitat or extinction of species. It is therefore necessary to assess whether species and habitats are protected in multiple sites across their full range, both to conserve genetic diversity and because species that exist on single or very few sites are vulnerable to unforeseen impacts or random events (such as extreme climatic events or pollution events).
3.1 Habitat quantity quality and connectivity
While good coverage is necessary, it is not sufficient. The Lawton Review's call for "bigger better and more joined up" areas in England is justified by ecological principles and therefore applicable also to Scotland and other countries, as habitat area, habitat quality, and habitat connectivity are widely regarded as key ingredients of any sound conservation policy (Lawton et al., 2010).
The size of core areas is related to the viability of their populations, as large areas can support larger populations, that are more buffered against unfavourable environmental variation, and also by having a reduced edge-effect. Large areas are also likely to support more species through the well-known species-area relationship (MacArthur and Wilson,1967).
While species of concern might be present in an area, it is unlikely that many of the existing terrestrial PAs, in particular small SSSIs are actually able to support viable populations of many species, i.e. populations for which, over the long term, births of individuals are as at least as numerous as deaths. When that is the case, ecologists talk about demographic sinks (Pulliam, 1988).
Habitat condition is also an important consideration and, especially in portions of landscape that are outside Protected Areas, this appears often insufficient to maintain healthy populations, given that many species groups are declining (see section 4).
Modelling work has shown that what drives viability at the landscape scale is not so much the existence of sinks but an insufficient number of sources, i.e. large enough areas in good condition (e.g. Gimona et al., 2011) where births outnumber deaths.
As to connectivity, it is important to note that not all types and sizes of vegetation patches between two areas constitute a corridor. Therefore while, for example, green networks made mainly for human recreation can also have a connecting function for some species, this is not necessarily the case for a number of species of conservation interest, unless a deliberate effort is made. Using the term ‘corridor’ in a rigorous sense would help clear misunderstanding about what an ecological network is, starting with whether all its components are actually in place (see Box 2).
A gap analysis could show the way forward in terms of improving coverage, and connectivity and point at the possible needs to enlarge some areas, to protect some new portions of the landscape and to create ecological corridors..
Finally, but crucially, despite the general principles, very little is known for many species of conservation interest about the functional connectivity provided by habitat networks. Example questions are: what is the adequate size of a core area? Is actual movement of propagules taking place in the network? What are the main barriers? What is the effect of interactions with other species? What is the potential role of networks in spreading pests and diseases? Ecological networks therefore could provide a good opportunity for detailed studies, relying, for example, on molecular genetics, new tracking technology, and traditional tagging methods, to examine for example how several “umbrella species” move during their life cycle and what keeps their populations viable. In the face of climate change, answering these questions is even more urgent (see below).
3.2 Marine Protected Areas
The Scottish Marine Protected Area network has benefitted from a coordinated and systematic approach to identifying the many Protected Areas and, while there were extensive stakeholder discussions, decisions on designation were not constrained by property rights as in their counterparts on land.
The network of MPAs established around the Scotland and the rest of the UK is based on an internationally agreed framework where limited biological knowledge about the spatial ecology of focal species and habitats has led to pragmatic attempts to protect known aggregations and ensure sufficient representation and replication at a regional sea level (see OSPAR ). To date MPAs cover over 30% of the Scottish sea area but measures within these areas that actually remove anthropogenic pressures are still at a fairly early stage, especially in offshore shelf areas. Consequently, there is the potential for further ecological evidence to guide the implementation of future measures. For a MPA network to be effective the spatial arrangement of protected patches across the network must be shown to promote population growth and ecosystem function in both the focal species and the wider protected communities, and ideally both within and outside the Protected Areas.
A major constraint in the development of the Scottish and UK MPA network has been the lack of ecological knowledge about the focal species that these areas are supposed to help conserve, as well as an absence of regular monitoring of their populations. Nevertheless, a growing body of research examining two key requirements; distribution and connectivity is helping to provide more relevant advice. Some species distribution models have suggested that the current distribution of focal priority species may be constrained by fishing activity (Stirling et al., 2016; Pinto et al., 2016) suggesting a need for restoration through targeted spatial restrictions on fishing effort. These distributional models have also been used to consider how climate change may affect aggregations currently found within MPAs (Fox et al., 2020). Biophysical models of connectivity (Gallego et al., 2017), together with new larval age data (Stirling et al., 2017) indicate that connectivity in species is linked to pelagic larval duration and may in some cases need to be improved by having shorter distances among protected aggregations.
As in the terrestrial case, the combination of biophysical and habitat suitability modelling (in addition to validation methods based on genetics or microchemistry) is a powerful tool to investigate the role of Protected Areas in the conservation status of species (Millar et al., 2019). For mobile species, estimates of active movements through a variety of geochemical and electronic tagging methods (Gibb et al., 2017; Neat et al., 2015) are providing an understanding of site fidelity of different life history stages and genetic studies are now being applied to estimate realised connectivity across a species’ range. Integrating these types of data into spatial models of population growth is the next step in providing advice on how to promote population growth of species and biodiversity in general. This may be through advice on the appropriate spatial arrangement and size of measures for the Scottish MPA network and/or other wider scale approaches to reduce the negative impact of anthropogenic pressures, such as technical approaches to reduce the by-catch of species at risk from bottom contact fishing gears (Valdemarsen et al., 2007; Kynoch et al., 2015).
3.3 Improving social networks and relationships
- to catalyse step changes needed for habitat connectivity and resilience
For PAs to be able to catalyse step changes in habitat connectivity and resilience there needs to be a recognition that PAs and their networks are not biophysical entities on maps. They are the outcomes of the interactions between governance structures, different actors and users, the natural resources and the overall system. These interactions, including feedbacks, occur at multiple levels and spatial scales, and are often nested in hierarchies. For ecological networks to be resilient and sustainable there needs to be a better alignment of the interactions between the scales and levels of the ecological systems (resources) and the different actors (land owners, nature conservation agencies, tourist) and the formal and informal rules-in-use (norms, policies, incentives). Scale mismatches between management actions and ecological systems can be spatial, temporal or functional, and include for example, when monitoring actions do not provide a whole system’s view of the problem or the PA network is not defined by the ecological network (Guerrero et al. 2013). It can be argued therefore, that opportunities for PAs to act as catalysts to promote habitat connectivity and resilience or solutions to integrate their management with wider landscapes need to be addressed through social dimensions.
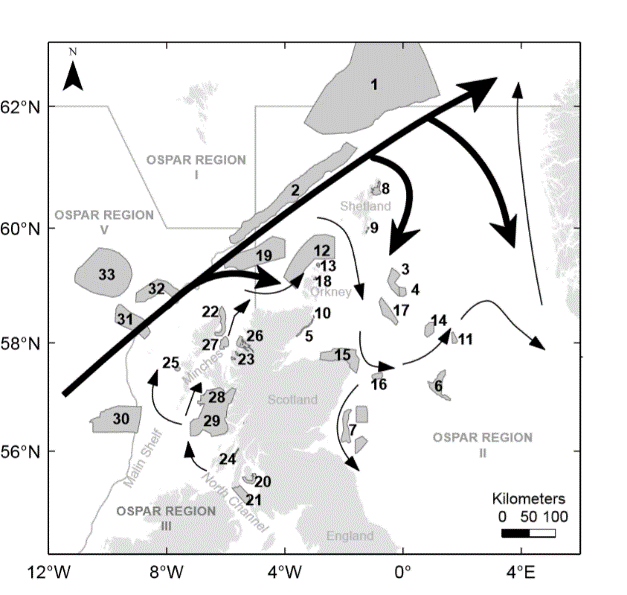
Distribution of the proposed Scottish Nature Conservation MPAs (numbered polygons) at the time the connectivity work of Gallego et al. (2017) was carried out, with an schematic outline of relevant residual currents in the area (thick arrows: Atlantic water; thin arrows: coastal water) and OSPAR (https://www.ospar.org) regional boundaries.
Current sea and land management practices are linked to a range of social dimensions and mechanisms, including formal and informal rules-in-use and norms. These rules and norms (including incentives) are social constructs which provide the means with which land managers use their ecological knowledge to produce a livelihood from resources, or deliver other ecosystem services such as recreation. This knowledge and the rules-in-use followed rely on social mechanisms for their generation, accumulation and transmission, and depend on social networks and relationship at different levels and scales (Folke et al 2007).
Box 2 - Ecological Corridors: Important principles
|
Ecological corridors (ECs) are not a substitute for core areas of adequate size. ECs are meant to complement and facilitate the ecological functioning of core areas by promoting functional connectivity. Connecting habitat fragments too small to be a population source for the species of interest is likely to be ineffective.
Ecological corridors in an ecological network should be of adequate size, have defined conservation objectives, and be managed accordingly
Ecological corridors should enjoy long term protection to ensure their continual function
Ecological corridors can encompass areas of landscape that are multifunctional in use, as long as this does not conflict with their primary objective, for example because of disturbance.
|
Therefore, it is the people and organisations that are involved in PA management and governance which can help align partnerships and collaborations to make better connections between PAs, their ecological networks, and connections to the wider landscapes. PA managers (at different levels in a nested governance hierarchy), in particular, are therefore critical in improving the interactions between the different actors involved and helping to develop the social networks that connect different sectors and organisations at different scales and levels (Eastwood et al, under review). In urban PAs, community rangers and engagement officers are a crucial link between the local community and reserve managers, allowing information flow between them. Individuals in PA social networks can act as knowledge brokers or bridging actors linking scales and actors that would be otherwise disconnected (Olsson et al. 2007). The exchange of ecological knowledge between different actors has been shown to be key in improving the management of, and building resilience of, socio-ecological systems (Olsson and Folke, 2001). For example, it was the exchange of ecological knowledge about individual cray-fishing and the whole watershed by different actors, fishermen and scientists that allowed Lake Racken (Sweden) fisheries to respond to the acidification threat of the lake (Olsson and Folke, 2001). This collective response was facilitated by a new fishing association (an adaptation of governance structures) that helped co-ordinate new management practices and the development of new rules-in-use. The accompanying learning that occurred (through monitoring and evaluation of decision making and actions) is a fundamental feature of adaptive management, and is key to adaptability and resilience.
There are examples of bridging actors, based on good relationships and trust, facilitating information and knowledge flows at different scales and levels in the Cairngorms National Park (e.g. Eastwood et al, under review).
Summary-Q2
Many PAs, by functioning as core areas can be a catalyst for the development of a set of ecological networks in Scotland. Large enough core areas in good habitat condition and functional corridors that allow movements of individual and genes are necessary components such networks. To expand coverage and to improve connectivity, corridors could be created or improved and there might be the opportunity to enlarge existing areas and to protect new ones However, while general principles are fairly well understood, substantial ecological knowledge gaps t need to be address to improve implementation on the ground.
Despite substantial needs to learn more about the species and habitats they help to conserve, Protected Areas are fundamental components of ecological networks both on land (including freshwater) and in the sea. However, it is not entirely clear that the existing portfolio of Protected Areas is part of functional networks. Filling these knowledge gaps is an important step towards achieving biodiversity targets.
Connectivity between ecological networks and the wider landscape could be promoted through improved social relationships and networks between different actors and sectors, for example between fisheries and nature conservation management. Trusted bridging actors and knowledge brokers, through their social networks can help better align (match) ecological networks with management actions and governance structures and facilitate more adaptive approaches.
Question 3
How best do we integrate the management of PAs with the wider land/marine management and use to achieve resilient ecological networks?
- Widening the view
4.1 Terrestrial landscapes
National-scale ecological networks are embedded in landscapes that have multiple land uses, and their role should be seen in the context of wider efforts to halt biodiversity loss, as it is essential to counter biodiversity loss at the right scale.
Biodiversity has continued to decline, not only internationally but also in Scotland (State of Nature Report, Scotland 2019). In principle, most governments are committed to its conservation. However, as the latest IPBES report documents, the opposite has occurred: habitat loss, fragmentation, pollution, unsustainable harvests have generally intensified, both in the period leading to 2010 and between 2010 and the present (2020). And climate change is already happening, with more changes looming large on the horizon.
In this context it has become increasingly clear that Protected Areas and their networks, despite their benefits (e.g. Naughton-Treves et al., 2011; Ferraro et al., 2011), are important but often insufficient, and a system approach is needed.
While trends are species-specific, the evidence for Scotland shows that 49% of species have declined in abundance, including many species of sea birds, which indicates that both the marine and terrestrial food chains might be disrupted and that protection of the basis of food chains outside designated areas is likely to be of key importance (Figure 4).
The observed vertebrate population declines, pollination shortfalls, declines in water purification capacity, the explosion of populations of herbivores are all likely to be aspects of weakening ecological interactions due to habitat destruction and probably also due to polluting agrochemicals such as neonicotinoids, known to affect non-target organisms and disrupt terrestrial and aquatic food chains . (e.g. Tooker, 2020; Yamamuro et al., 2019; Douglas et al., 2015; Pisa et al., 2015).
The effects of human activities related to harvesting wild populations over large areas, both on land and in the sea, are also clearly relevant, and their effects have been under scrutiny for years. Such activities also rely on ecosystem functioning.
In Scotland the Edinburgh Declaration appears to commit sub-national governments to action, This is, no doubt, well intentioned, but it will be the quality of legislation passed , the level of coordination, the involvement of land managers, and the resources allocated that will influence biodiversity trends.
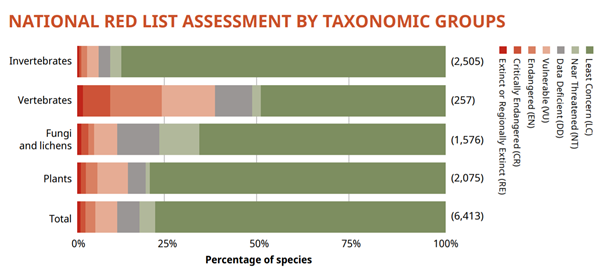
Scottish species assessment. Source: State of Nature Report Scotland 2019.
Ecological Networks as Part of the Wider Landscape
Ecological networks will have to be part of wider landscape management. However the solutions that scientists can offer are only partial, because ecological theory cannot solve the problem of competing land and sea uses, embedded in world-wide commodity chains, and part of a system that both satisfies and creates consumer demands.
Most scientists agree that conservation efforts are needed at multiple spatial scales (e.g. Poiani et. al., 2000; Lindenmayer et al., 2006; Sodhi et al., 2011; Bonebrake et al., 2019) and that the regional and national scales need to be addressed. For example, a well designed National Ecological Network would certainly contribute to this goal.
Ecologists have debated whether conservation areas should or should not be segregated from productive activities and have called land sparing and land sharing two opposite strategies proposed for biodiversity protection. Land sparing suggests that trade-offs between productive activities and biodiversity can be better alleviated through spatial segregation of conservation and productive land uses, which would result in higher productivity and therefore in more land that could be devoted to conservation elsewhere (e.g. Green et al., 2005; Phalan et al., 2011). Land sharing suggests that productive and conservation uses could be interspersed at a relatively fine scale by de-intensifying production. The definition also depends on scale. Here we talk about land sparing and sharing over areas of 10s to 100s of kilometres.
After the Millennium Ecosystem Assessment, followed by a number of national assessments, awareness of the need for a holistic approach to landscape management has grown in society, together with the recognition that multiple benefits should be protected to enhance human wellbeing. There is high-level scientific consensus, fostered by IPBES, that the interdependence between ecosystem functioning and biodiversity calls for a systemic approach in designing effective policies addressing multiple aspects at the same time and encompassing all levels of “naturalness”. Decisive and spatially targeted action is considered crucial (Diaz et al., 2020). This implies embedding a well-resourced biodiversity conservation policy within multifunctional landscapes, and ‘making space for nature’ with ‘nature-based solutions’ delivering multiple benefits.
Intrinsic Value and Utility Value of Nature
However, the conservation discourse often mixes, quite liberally, two ethical paradigms that, despite the rhetoric, are not always compatible: one based on the intrinsic value of biodiversity and one based on its utilitarian value for people. The clash becomes evident, for example, when an (explicit or implicit) cost-benefit analysis indicates that nearly irreplaceable ancient woodlands, which are crucial parts of a UK-wide ecological network, are expendable (or indeed movable) to make space for transport infrastructure.
This is an example of the fact that generally, scientific advice notwithstanding, most choices can only be made with reference to values, such as: should species / habitats be protected from disturbance or be integrated into multifunctional green networks that are as good for recreation as for environmental protection? The latter is biased towards human use, excluding species that don’t tolerate disturbance very well or are subject to increased predation due to edge effects
As to values, it is conceivable to integrate biodiversity and ecosystem services by making a process similar to that in Figure 5 (below) participatory and open to a wide range of stakeholders. However, the contribution of scientists is important. Care needs to be taken to select the important elements. The question of comprehensiveness of a portfolio of valuable and functioning areas also remerges in the wider, non protected landscape: preserving landscape heterogeneity and reducing land-use intensity is often advocated, and maintaining heterogeneity at the landscape and at the regional level is believed to enhance biodiversity protection over large areas.(e.g. Benton et al., 2003; Tscharntke et al., 2012).
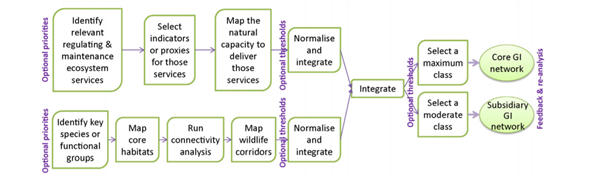
Approach to integrate ecological networks and ecosystem services. Source: Liquete et al., 2015.
Figure 5 . Approach to integrate ecological networks and ecosystem services. Source: Liquete et al., 2015.
Most studies seeking to reconcile biodiversity conservation with agricultural production have found land sparing to be a better solution (see e.g., von Wehrden et al., 2014; Lamb et al., 2019). When considering other environmental aspects, such as pollution affecting the ecological status of freshwaters, or the risk of flooding, we can make similar considerations. A critical minimum amount of semi-natural features is needed to ensure benefits.
Segregating production and conservation in agricultural landscapes might often be difficult in practice. However, the expansion of ‘High Nature Value” (HNV) farmlands from existing ‘core’ example areas could be one of the courses of action to take, with significant protection gaps to fill in the lowlands. As pointed out by Lomba et al.( 2020), this would require a system approach that goes well beyond the simple, but necessary, incentives, by empowering land managers and rural communities through capacity building, business diversification, promotion of HNV products, and provision of technology. Restoring peatlands over large areas and planting networks of native woodlands are also priority actions.
Reconnection of urban and rural environment would also be important. However, the planning of networks of semi-natural areas that aim to deliver a wide range of ecosystem services such recreation, water purification, air quality would need to address explicitly functional connectivity to qualify also as an ecological network. These networks composed of green and blue (water) are extremely valuable for people’s health and well-being, and, if carefully conceived, can also enhance biodiversity protection.
Freshwater ecosystems
Finally, freshwater ecosystems would deserve much more space that we can afford. In brief, they are clearly influenced by land use management in their catchment, so landscape-level policy is important. And catchment management plans could integrate with regional and national-scalenetworks. The connectivity restoration aspect specific to freshwater, however, is addressed by the removal or bypassing of infrastructure that disrupts movements of water, species, sediments, and nutrients (Grill et al. 2019).
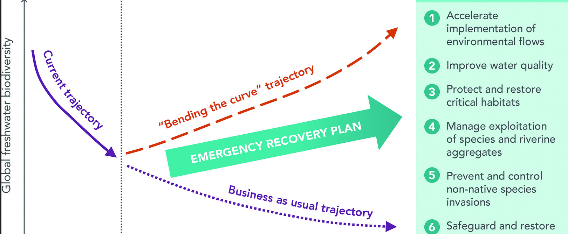
Connectivity in a wider context for freshwater ecosystems. From Tickner et al., 2020
All of the above suggest that land sparing and sharing at multiple scales are needed with a strategy comprising wide-scale restoration areas and local-scale habitat restored, created or protected to contribute to the demographic viability of the species of interest, either as breeding habitat or as a dispersal conduit. The range of interventions would go from re-wilding several thousand square Km in the uplands to improving landscape conditions in selected areas that cover several hundred square kilometres each.
Returning to governance, it is now, hopefully, evident that governance has to match the scale of processes. It is unlikely that this could be achieved without deliberate spatial planning that provides a form of landscape prioritisation. While bottom-up initiatives are essential (see also section 4.4 below) and very effective at building social capital among participants, they might lack the coordination and overall vision needed to achieve conservation of biodiversity at a scale that matters.
New institutions, able to facilitate the complementarity of initiatives and effective targeting of public money are needed. Regional Land Use Partnerships (RLUPs) are a potential candidate. The interim report about the newly created RLUPs is encouraging in his statement of principles and goals, but it is unclear, at this stage, whether they will have real power. Also, coordination between regional partnerships would be essential to achieve national-scale objectives.
The payments of spatially targeted incentives to land managers is potentially a powerful instrument. Given the very substantial amount of public money is in principle available to fund the successor of the Common Agricultural Policy (CAP) in the UK, there is an opportunity to re-design and re-orient payment substantially towards public goods in the post-CAP era, to enhance biodiversity protection at the landscape level. For example there could be a requirement to devote land to conservation to receive support; and areas of landscape devoted to increasing connectivity could be given special priority. Landscape conservation areas could also be identified and prioritised to incentivise the maintenance and creation of high nature value farmland.
Bottom-up approaches are, nonetheless, fundamental too. Putting conservation principles into practice to form a National Ecological Network is not necessarily easy given competing pressures and points of view. Conservation at the landscape level implies that not only communities but also private landowners will have to play an important role and be persuaded to long-term commitments.
BOX 3 Key opportunities to improve the use of incentives for biodiversity conservation.
|
-Aim to restore large portions of landscape (100s to 1000s square Km) -Pay incentives mainly for public goods, but as part of a wider socio-ecological strategy. -Promote public participation in setting landscape-level objectives to be incentivised -Promote effective collaborative implementation of landscape‐level measures -Promote spatial planning to coordinate collaborative measures and partnership initiatives -Strengthen environmental monitoring and tie payments to both activities and outcome
|
4.2 Marine Protected Areas in the wider marine management context
The Scottish Government has a three pillar approach to marine conservation; species conservation, site protection and wider seas policies and measures). For example, for the critically endangered flapper skate it is both illegal to land this species and there is an MPA designated to protect a mostly resident group (Neat et al., 2015). However, marine management has tended to maintain a distinction between fishery and nature conservation measures and this is evident in Scotland from the different public bodies advising on sustainable fisheries and nature conservation and how these interests are represented through management and government all the way to ministerial level. This distinction is not consistent with international treaties that have expounded the ecosystem approach or is consistent with promoting resilient ecological networks. Therefore, we have not reached the point where integrated ecosystem assessments are used in marine management advice. While not integrated, assessments under the UK Marine Strategy do provide an indication of the current state of different biota. Based on these latest assessments Good Environmental Status (GES) has not been achieved for most seafloor habitats with the state of sediment habitats being uncertain. As commercial fishing is the predominant human pressure preventing GES being achieved in seafloor habitats, joint consideration of fisheries management and marine conservation objectives may help to improve marine management.
In terms of site protection, greater consideration could be given to target site protection measures in locations where both species of nature conservation concern and habitat of importance to commercial fish and shellfish occur. Unfortunately, measures to protect focal species within MPAs are generally seen as detrimental to the fishing industry. Consequently, ways to target future measures that best promote population growth and resilience of focal species and their associated communities, while minimising impacts on fisheries are needed. Spatial management can increase fishery profits relative to non-spatial management, if protected areas are strategically placed to promote connectivity and population growth in target species (Rassweiler et al., 2012). However, the use of protected (closed) areas in fishery management has generally been seen to have failed because of a lack of clear goals against which success could be evaluated and the implementation of such measures when a population is close to collapse (e.g. Clark et al., 2015).
Many of the species and habitats that are focal features of MPAs have the potential to provide essential services for productive fisheries (Armstrong and Falk-Petersen, 2008). With habitat diversity promoting population abundance for benthic species e.g. Sundblad et al., 2013; Lilley and Unsworth, 2014; Elliot et al., 2017; Hancock and zu Ermgassen, 2019).
Protected areas, in addition to restoring the habitat of some commercial species, can also reduce mortality on some resident targeted species, such as lobsters and scallops which should eventually lead to overspill into surrounding areas. This overspill may lead to a net gain in fishery yields in some cases (Di Lorenzo et al., 2016).
Displacing fishing effort from MPAs may lead to more extensive environmental damage and a net damage to ecosystem processes (Greenstreet et al., 2009). As the first pass of mobile gear can have an impact on benthic communities (Cook et al., 2013; Hiddink et al., 2006), prohibiting fishing from areas where it occurs at low intensity, rather than highly used areas, can lead to a large reduction in the total footprint of fishing, with less impact on fishing industry and displacement to previously unfished areas (Dinmore, 2003). Such an approach may produce the greatest ecological benefit at the regional scale for the lowest economic cost.
In summary, further management of MPAs needs to consider the spatial processes governing resilience and how measures could benefit both the focal features important to nature conservation as well as promoting the productivity of commercial fish and shellfish stocks. This could be facilitated using scenario modelling platforms such as Marxan Connect, which can integrate the growing spatial and connectivity evidence base with costs associated with protection (Daigle et al., 2020). Conversely, wider marine (including fisheries) management that promotes a healthier marine environment (e.g. through a wider size/age structure of the population) would make the marine populations outside and within protected areas more resilient to anthropogenic (e.g. climate change, invasive species, pollution) and even natural (e.g. resulting from natural variability) stressors.
4.3 The impact of climate change
To be resilient, ecological networks comprising protected areas will have to deal with climate change. Thanks to the invaluable evidence provided by monitoring, we know that the impacts of climate change are already visible and rather widespread in the UK. While monitoring data should be seen in the context of multiple pressures (e.g. Ceballos et al., 2015), impacts and knowledge gaps are well documented. For example in the UK terrestrial and marine report cards and in scientific papers showing that a range of taxa are responding, often negatively. A good example is a study by Martay et al.(2017), who show that populations of a range of invertebrates, birds and mammals are responding to climate signals, some with a marked decline. Also, multiple taxa already show a range expansion towards the north. In the marine environment several climate change stressors including rising temperatures, ocean acidification, changes in ocean currents and wave exposure are all expected to affect the future extent of suitable habitat and connectivity.
Given the projections of climate models, in the next decades pressure is likely to intensify on phenology, geographic distribution and population dynamics. And given that the UK has experienced less than 1 oC warming so far, it is reasonable to expect much stronger impacts than observed to date, especially if warming soars to 3 oC or more, as implied by the present international GHG emission reduction pledges (Nationally Defined Contributions) associated with the Paris Agreement.
There are likely to be both winners and losers, but the expectation from a range of studies is that impacts could be significant on several taxa (Thomas et al 2004; Chen et al., 2011). While management could mitigate impacts, protected areas might well see the decline of their features of interest; and understanding of which species and habitats are at risk is important. These considerations apply across the terrestrial and marine environment.
A comprehensive risk assessment for terrestrial taxa has been provided by Pearce-Higgins et al., (2017) who showed that upland species were most at risk, and that of the 402 species examined, 35% were likely to suffer range contraction and 42% likely to undergo range expansion.
Adaptation
The above evidence points to three interconnected strategic needs: (i) to increase the resilience of existing habitats and populations when the impacts appear moderate; (ii) to increase landscape/seascape permeability to allow range shift; and: (iii) to prepare to manage inevitable change, for example by welcoming new species that are moving northwards from other areas of Europe where, in some cases, climatic conditions might be no longer viable. National-level ecological networks could play a crucial role (see also Thomas, 2020).
Finally, often overlooked, in Scotland and elsewhere is the potential effect of climate-induced land use change. Land use change remains the main threat to biodiversity (ref) and its interaction with climate change can reinforce the threat. The likely (at least initial) improvement in capability for agriculture and forestry in Scotland (Brown et al., 2011) in some areas under projected climate change. This might generate new conflicts that would be better to minimise through spatial planning (e.g. Gimona et al., 2015). In general, the area of future interactions between land use change and climate is under-investigated (to Bühne et al., 2020), and needs to be better understood to adapt and harmonise policy.
The potential effects of climate change on fisheries may require substantial technical adaptation from fisheries and public support to cope with associated changes in fish community composition and increased storminess (Pinnegar et al., 2020). The later may lead to increased pressure on more sheltered marine areas. Aquaculture may also have to adapt as the environment becomes less suitable for salmon culture and more prone to harmful algal blooms. These changes further highlight the need for the growing focus on marine spatial planning, including the siting of MPAs.
Because climate change is a threat multiplier, reducing other pressures could increase the resilience of species and habitats within and outside PAs (e.g. Oliver et al., 2015).
Oliver et al. (2015) provide a comprehensive species-centred framework for adaptation reproduced below. To take advantage of the ecological network concept, by identifying a number of umbrella species in the studies mentioned so far, it would be possible to make those recommendations spatially explicit by mapping where in the landscape the actions suggested could occur (see also Oliver et al., 2016 for an application of the framework in Table 1). For example: (i) where landscape restoration could be prioritised; (ii) where functional and well-designed ecological corridors to improve connectivity between core areas could be placed to allow range shift; (iii) where new PAs could be created given modelled climatic range shifts; (iv) where there might be future conflicts with expanding land and marine uses such as agriculture, forestry the developing aquaculture industry and marine renewable developments.
Ecological Networks and Climate Change
Because ecological networks are not a panacea, it would be useful to identify important species which are unlikely to be able to shift their distribution (trailing edge of the range) to keep pace with climatic change (Brooker et al., 2017; Gimona et al., 2012). This could aid the triage process and allow conservation managers to consider whether translocation is appropriate.
All of the above reinforces the principle that it is essential to have a joined-up land-, and sea- use policy at the national level, of which conservation is an element.
Despite all the excellent suggestions provided by several authors, while principles are a useful starting point, an adaptive approach dealing with the peculiarities of each case will often be needed. Therefore the interdisciplinary science and art of change management will have to develop significantly in the next few decades, and the need to regard landscapes and their ecological networks as part of social-ecological systems will come into even sharper focus to help biodiversity conservation.
| Adaptation Action | Description | Purpose |
|---|---|---|
|
Buffer (edge impacts) |
Manage buffer zone around existing habitat patches to reduce negative impacts from their surroundings (e.g. chemical drift from intensive farming, hunting). |
Increase population size, propagule pressure and popn. resilience. |
|
In‐situ management |
Manage and protect existing habitat to improve habitat quality; conserve heterogeneity; and reduce or remove other non‐climate related threats (e.g. over‐grazing). |
Increase popn. size, propagule pressure and popn. Resilience. |
|
Restore (expand/nearby/other sites) |
Restore degraded, or create new, habitat adjacent to existing suitable sites (expand), nearby occupied sites or at other sites. Restore or create habitat across environmental/elevational/geographical gradients to increase habitat area; increase ecological variability; improve connectivity; and improve representation and replication of habitats and ecosystems. |
Increase popn. size, propagule pressure and local and regional‐scale popn. resilience. Improve opportunities for species to adjust distributions in response to climate change. |
|
Creats (expland/nearby/other sites) |
Restore degraded, or create new, habitat adjacent to existing suitable sites (expeand), nearby occupied sites or at other sites. Restore or create habitats across environmental/elevational/geographical gradients to increase habitat area;increase ecological variability;improve connectivity; and improve representation and replication of habitats and ecosystems
|
Increase popn size, propagule pressure and local and regional-scale popn resilience. Improve opportunities for species to adjust distributions in response to climate change. |
|
Manage matrix |
Protect, manage, restore, create features in the landscape that promote species movement, for example, less intensive farming, urban green space. |
Improve opportunities for species to adjust distributions in response to climate change. Increase regional‐scale popn. resilience. |
|
Translocate |
Augment current species’ popns. with individuals from other areas, or aid colonization of species into new areas through transfer of individuals from existing source popns. |
Increase popn. resilience and create colonization of new patches. |
|
Ex‐situ |
Establish captive popns. of species that would otherwise become extinct due to climate change. |
Conserve species. |
|
Accept loss |
Take no action to conserve a species where there appears to be no reasonable course of action that will ensure its continued existence in the locality. |
Conserve resources where they would be better directed elsewhere. |
|
Monitor & research |
Monitor the success of conservation actions and improve understanding of the impact of climate change on viability and resilience of species, communities, habitats and ecological processes through research. |
Inform adaptive management and choice of future actions. |
4.4 Improving alignment/adaptability of management and governance systems with ecological networks
Socio-ecological systems are dynamic and vary with time, being susceptible to disturbances such as fire, pest outbreaks and diseases. Ecological networks are therefore vulnerable to these changes and disturbance and often these occur in non-linear and unpredictable ways. Just as ecological systems need to be resilient to changes, buffer against shocks, and respond to feedback, so do the social-ecological systems they are part of. This resilience, however, is based around the ability of governance and management structures and associated institutions to adapt and change, and be responsive to environmental and social feedbacks. They can only do so effectively if the management and governance institutions (operating at different scales and levels) are: (i) aligned with the ecological systems and processes; and: (ii) flexible enough to respond to feedback (environmental and social). Often it is a mismatch of scales between ecological structures and processes and social structures and processes that are in place to manage and govern resources that can lead to the degradation of ecosystems, and ultimately ecological networks. Flexibility and adaptability of management practices to respond to shocks and disturbance that may threaten ecological networks, or more correctly social-ecological ones, will be dependent on having the governance frameworks and institutions that can also adapt to new social, economic and ecological knowledge as they arise. Improving social networks and relationships, can be achieved e.g. through co-constructed monitoring, and by having governance structures across multiple scales and levels (i.e. link fisheries management with nature conservation). This would allow different ecological and social knowledge to be monitored and evaluated in an integrated way, and lead to more responsive management (learning-by-doing) at appropriate scales. Research has shown that having highly fragmented social networks led to a polarisation of norms in practice, and were a barrier to adaptive co-management of small-scales fisheries with consequences for marine reserves (Alexander et al. 2015)
Other researchers (Maciejewski & Cumming, 2015) have also described the parallels between ecological networks and corridors, contributing to ecological resilience, and how social interactions and networks contributing indirectly through actor learning and adaptive management. In their study on several hundred South African PAs these authors recommended deliberately fostering particular socio-economic corridors (social networks) to make PAs more resilient to perturbations, such as fires and disease outbreaks. Another step where PAs to act as catalysts for habitat connectivity and resilience is to also build and develop trusted PA social networks and relationships (see previous section) between different governance structures and sectors.
Summary -Q3
Ecological networks can be made more resilient if managed in the context of the landscapes and seascapes that they are part of. This could be done by re-orienting incentives, promoting public participation and by matching governance and planning to the scale of the biophysical processes that we want to protect. Flexibility and adaptability of institutions and management practices would add to the ability to deal with environmental and societal change.
Functioning networks could be a central component of sustainable harvest policies, conservation policies, and also adaptation policies that increase biodiversity resilience to climate change.
References
Alexander, S. M., Armitage, D., and Charles, A. 2015. Social networks and transitions to co-management in Jamaican marine reserves and small-scale fisheries. Global Environmental Change-Human and Policy Dimensions, 35: 213-225.
Allen J., Massey, D., and Cochrane, A. 1998. Rethinking the Region. London, Routledge.
Armstrong, C.W. and Falk-Petersen, J., 2008. Habitat–fisheries interactions: a missing link? ICES Journal of Marine Science, 65 6.
Bennett, G., Wit, P. 2001. The Development and Application of Ecological Networks A Review of Proposals, Plans and Programmes. AIDEnvironment, Amsterdam.
Benton, T. G., Vickery, J. A., and Wilson, J. D. 2003. Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evol. 4, 182–188. doi: 10.1016/S0169-53470300011-9
Bonebrake,T.C., Guo,F., and Dingle,C. et al.,2019. Integrating proximal and horizon threats to biodiversity for conservation. Trends in ecology & Evolution, 34, 781-88.
Brooker, R. W., Brewer, M. J., Britton, A. J., Eastwood, A., Ellis, C., Gimona, A., Poggio, L., et al. 2018. Tiny niches and translocations: The challenge of identifying suitable recipient sites for small and immobile species. Journal of Applied Ecology, 55: 621-630.
Brown, I., Poggio, L., Gimona, A., and Castellazzi, M. 2011. Climate change, drought risk and land capability for agriculture: implications for land use in Scotland. Regional Environmental Change, 11: 503-518.
Broekmeyer, M. and Steingröver, E., eds. 2001 Handboek Robuuste Verbindingen (Handbook for ecological corridors). Alterra, Wageningen.
Ceballos, G. et al. 2015. Accelerated modern human–induced species losses: entering the sixth mass extinction. – Sci. Adv. 1: e1400253.
Ceballos, G., Ehrlich, P. R., Barnosky, A. D., Garcia, A., Pringle, R. M., and Palmer, T. M. 2015. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Science Advances, 1.
Chen, I. C., Hill, J. K., Ohlemuller, R., Roy, D. B., and Thomas, C. D. 2011. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science, 333: 1024-1026.
Clarke, J., Bailey, D. M., and Wright, P. J. 2015. Original Article: Evaluating the effectiveness of a seasonal spawning area closure. ICES Journal of Marine Science, 72: 2627–2637. https://doi.org/10.1093/icesjms/fsv144.
Cook, R., Fariñas-Franco, J.M., Gell, F.R., Holt, R.H.F., Holt, T., Lindenbaum, C., Porter, J.S., Seed, R., Skates, L.R., Stringell, T.B., Sanderson, W.G., 2013. The Substantial First Impact of Bottom Fishing on Rare Biodiversity Hotspots: A Dilemma for Evidence-Based Conservation. PLoS ONE 8, e69904. https://doi.org/10.1371/journal.pone.0069904
Cumming, G. S., and Allen, C. R. 2017. Protected areas as social-ecological systems: perspectives from resilience and social-ecological systems theory. Ecological Applications, 27: 1709-1717.
Daigle, R. M., Metaxas, A., Balbar, A. C., McGowan, J., Treml, E. A., Kuempel, C. D., Possingham, H. P., et al. 2020. Operationalizing ecological connectivity in spatial conservation planning with Marxan Connect. Methods in Ecology and Evolution, 11: 570–579. John Wiley & Sons, Ltd. https://doi.org/10.1111/2041-210X.13349.
De Vos, A., Cumming, G. S., et al. 2016. Pathogens, disease, and the social-ecological resilience of protected areas. Ecology and Society, 21.
Di Lorenzo, M., Claudet, J., and Guidetti, P. 2016. Spillover from marine protected areas to adjacent fisheries has an ecological and a fishery component. Journal for Nature Conservation, 32: 62–66. http://www.sciencedirect.com/science/article/pii/S1617138116300255.
Diaz et al., 2020. Set ambitious goals for biodiversity and sustainability. Science, 370,6515,411-413 doi: 10.1126/science.abe1530
Dinmore, T., 2003. Impact of a large-scale area closure on patterns of fishing disturbance and the consequences for benthic communities. ICES Journal of Marine Science 60, 371–380. https://doi.org/10.1016/S1054-31390300010-9
Douglas, M.R., Rohr, J.R., Tooker, J.F., 2015. Neonicotinoid insecticide travels through a soil food chain, disrupting biological control of non-target pests and decreasing soya bean yield. Journal of Applied Ecology 52, 250-260.
Eastwood, A., Fischer, A., Hague, A, and Brown, K. under review A cup of tea? - The role of social relationships, networks and learning in land managers’ adaptation to policy change. Under review in Land Use Policy.
Ferraro, P.J.,Hanaue, M.M. Sims, K.R.E. 2011. Conditions associated with protected area success in conservation and poverty reduction PNAS, 108, 13913-13918; https://doi.org/10.1073/pnas.1011529108
Folke, C., Pritchard, L., ..and Svedin, U. 2007. The problem of fit between ecosystems and institutions: Ten years later. Ecology and Society, 12.
Fox, A. D., Henry, L.-A., Corne, D. W., and Roberts, J. M. 2020. Sensitivity of marine protected area network connectivity to atmospheric variability. Royal Society Open Science, 3: 160494. Royal Society. https://doi.org/10.1098/rsos.160494.
Frank, S.D., Tooker, J.F., 2020. Opinion: Neonicotinoids pose undocumented threats to food webs. Proceedings of the National Academy of Sciences of the United States of America 117, 22609-22613.
Gallego, A., Gibb, F. M., Tullet, D., and Wright, P. J. 2017. Bio-physical connectivity patterns of benthic marine species used in the designation of Scottish nature conservation marine protected areas. ICES Journal of Marine Science, 74: 1797–1811. https://doi.org/10.1093/icesjms/fsw174.
Gibb, F. M., Régnier, T., Donald, K., and Wright, P. J. 2017. Connectivity in the early life history of sandeel inferred from otolith microchemistry. Journal of Sea Research, 119: 8–16. http://www.sciencedirect.com/science/article/pii/S1385110116301022.
Gimona, A., Poggio, L., Brown, I., and Castellazzi, M. 2012. Woodland networks in a changing climate: Threats from land use change. Biological Conservation, 149: 93-102.
Gimona, A., Poggio, L., Polhill, J. G., and Castellazzi, M. 2015. Habitat networks and food security: promoting species range shift under climate change depends on life history and the dynamics of land use choices. Landscape Ecology, 30: 771-789.
Gimona, A., Polhill, J.G. and Davies, B. 2011. Sources , Sinks and Conservation Incentives. in Sources, Sinks and Sustainability. Liu et al. eds. 2011, CUP.
Green,R.E.,Cornell,S.J.,Scharlemann,J.P.W.,andBalmford,A.2005.Farming and the fate of wild nature. Science 307, 550–555. doi: 10.1126/science.1106049
Greenstreet, S. P. R., Fraser, H. M., and Piet, G. J. 2009. Using MPAs to address regional-scale ecological objectives in the North Sea: modelling the effects of fishing effort displacement. ICES Journal of Marine Science, 66: 90–100. https://doi.org/10.1093/icesjms/fsn214.
Grill,G., Lehnet, B. .. Zarfl, C. 2019.. Mapping the world's free-flowing rivers. Nature 569, 215–221.
Guerrero, A. M., McAllister, R. R. … and Wilson, K. A. 2013. Scale Mismatches, Conservation Planning, and the Value of Social-Network Analyses. Conservation Biology, 27: 35-44.
Hackett B., 1964 The landscape content of the plan, Planning Outlook, 6:2,5-
Hancock B., zu Ermgassen P., 2019. Enhanced Production of Finfish and Large Crustaceans by Bivalve Reefs. In: Smaal A., Ferreira J., Grant J., Petersen J., Strand Ø. eds Goods and Services of Marine Bivalves. Springer, Cham
Hanksi, I. and Gilpin, M. 1997. Metapopulation Biology: Ecology, Genetics and Evolution, Academic Press, London.
Hidalgo, M., Secor, D. H., and Browman, H. I. 2016. Observing and managing seascapes: linking synoptic oceanography, ecological processes, and geospatial modelling. ICES Journal of Marine Science, 73: 1825–1830. https://doi.org/10.1093/icesjms/fsw079.
Hiddink, J.G., Jennings, S., Kaiser, M.J., Queirós, A.M., Duplisea, D.E., Piet, G.J., 2006. Cumulative impacts of seabed trawl disturbance on benthic biomass, production, and species richness in different habitats. Canadian Journal of Fisheries and Aquatic Sciences 63, 721–736. https://doi.org/10.1139/f05-266
Kaufmann, M. R., Graham, R.T., ..and Corn, P.S. 1994. An ecological basis for ecosystem management. U.S. Forest Service General Technical Report RM-GTR-246, Rocky Mountain Research Station, Fort Collins, Colorado, USA.
Kritzer, J., and Sale, P. 2006. Marine Metapopulations. Academic Press, Burlington. http://www.sciencedirect.com/science/article/pii/B9780120887811500017.
Kynoch, R. J., Fryer, R. J., and Neat, F. C. 2015. A simple technical measure to reduce bycatch and discard of skates and sharks in mixed-species bottom-trawl fisheries. ICES Journal of Marine Science, 72: 1861–1868. https://doi.org/10.1093/icesjms/fsv037.
Langton, R., Stirling, D. A., and Wright, P. J. 2020. Are MPAs effective in removing fishing pressure from benthic species and habitats? Biological Conservation, 247: 108511. http://www.sciencedirect.com/science/article/pii/S0006320719312030.
Lawton, J. H., Brotherton, … Wynne, G. R. 2010. Making space for nature: A review of England's wildlife Sites and ecological network. Report to Defra, 107.
Levins, R. 1969 Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull. Entomol. Soc. Amer. 15 237–240
Lilley, R.J.; Unsworth, R.K.F., 2014. Atlantic Cod Gadus morhua benefits from the availability of seagrass Zostera marina nursery habitat. Global Ecology and Conservation 2: 367–377.
Lindenmayer,D.B., Franklin, J.F., Fischer, J. 2006. General management principles and a checklist of strategies to guide forest biodiversity conservation Biological Conservation, 131, 433-445
Liquete, C., Kleeschlute, S...Zulian, G. 2015. Mapping green infrastructure based on ecosystem services and ecological networks: A Pan-European case study. Environmental Science & Policy,54, 268-280
Lomba, A., Moreira, F., …and McCracken, D. I. 2020. Back to the future: Rethinking socioecological systems underlying high nature value farmlands. Frontiers in Ecology and the Environment, 18, 36– 42. https://doi.org/10.1002/fee.2116
MacArthur, R. H., & Wilson, E. O. 1967. The theory of Island biogeography. Princeton University Press.
Maciejewski, K., and Cumming, G. S. 2015. The relevance of socioeconomic interactions for the resilience of protected area networks. Ecosphere, 6:14.
Margules, C. R. & Pressey, R. L. Systematic Conservation Planning.Nature 405, 243–253 2000.
Martay, B., Brewer, M. J., Elston, D. A., Bell, J. R., Harrington, R., Brereton, T. M., Barlow, K. E., et al. 2017. Impacts of climate change on national biodiversity population trends. Ecography, 40: 1139-1151.
McGinnis, M. D., and Ostrom, E. 2014. Social-ecological system framework: initial changes and continuing challenges. Ecology and Society, 19: 12.
Millar, Hannah, Rory O’Hara Murray, Alejandro Gallego, Kate Gormley and Flora Kent. 2019. Connectivity of selected Priority Marine Features within and outwith the Scottish MPA network. Scottish Natural Heritage Commissioned Report No. 1048
Mizerovsky, G. J. 1963 Types of plans of rural settlements in Bohemia and Moravia-Silesia: A study in human geography
Naughton-Treves, L., Alix-Garcia, J. and Chapman, C. 2011.Lessons about parks and poverty from a decade of forest loss and economic growth around Kibale National Park, Ugand. PNAS, 108, 13919-13924; https://doi.org/10.1073/pnas.1013332108
Naveh,Z. 1982 Landscape Ecology as an Emerging Branch of Human Ecosystem Science. Advances in Ecological Research, 12,189-237.
Neat, F., Pinto, C., Burrett, I., Cowie, L., Travis, J., Thorburn, J., Gibb, F., et al. 2015. Site fidelity, survival and conservation options for the threatened flapper skate Dipturus cf. intermedia. Aquatic Conservation: Marine and Freshwater Ecosystems, 25: 6–20. John Wiley & Sons, Ltd. https://doi.org/10.1002/aqc.2472.
Oliver, T. H., Smithers, R. J., Bailey, S., Walmsley, C. A., and Watts, K. 2015. A decision framework for considering climate change adaptation in biodiversity conservation vol 49, pg 1247, 2012. Journal of Applied Ecology, 52: 538-538.
Oliver, T. H., Smithers, R. J., Beale, C. M., and Watts, K. 2016. Are existing biodiversity conservation strategies appropriate in a changing climate? Biological Conservation, 193: 17-26.
Olsson, P., and Folke, C. 2001. Local ecological knowledge and institutional dynamics for ecosystem management: A study of Lake Racken Watershed, Sweden. Ecosystems, 4: 85-104.
Olsson, P., Folke, C., Galaz, V., Hahn, T., and Schultz, L. 2007. Enhancing the fit through adaptive co-management: Creating and maintaining bridging functions for matching scales in the Kristianstads Vattenrike Biosphere Reserve, Sweden. Ecology and Society, 12.
Ostrom, E., Janssen, M. A., and Anderies, J. M. 2007. Going beyond panaceas. Proceedings of the National Academy of Sciences of the United States of America, 104: 15176-15178.
Pearce-Higgins, J. W., Beale, C. M., Oliver, T. H., August, T. A., Carroll, M., Massimino, D., Ockendon, N., et al. 2017. A national-scale assessment of climate change impacts on species: Assessing the balance of risks and opportunities for multiple taxa. Biological Conservation, 213: 124-134.
Phalan, B., Onial, M., Balmford, A., and Green, R. H. 2011. Reconciling food production and biodiversity conservation: land sharing and land sparing compared. Science 333, 1289–1291. doi: 10.1126/science.1208742
Pinnegar, J.K., Wright, P.J., Maltby, K. and Garrett, A. (2020) Impacts of climate change on fisheries relevant to the coastal and marine environment around the UK. MCCIP Science Review 2020, 456–481.
Pittman S.J. (Editor) (2017) Seascape Ecology, Wiley-Blackwell 528pp, ISBN: 978-1-119-08444-0
Pisa, L.W., Amaral-Rogers, V., ..and Wiemers, M., 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environmental Science and Pollution Research 22, 68-102.
Poiani, K., Richter, B.D., Anderson,M.G., Richter, H. E. 2000. Biodiversity Conservation at Multiple Scales: Functional Sites, Landscapes, and Networks. BioScience, 50, ,133–146.
Proctor, R., Wright, P., and Everitt, A. 1998. Modelling the transport of larval sandeels on the north west European shelf. Fisheries Oceanography 7: 347-354.
Pulliam, H.R. 1988 Sources, sinks and population regulation. American Naturalist, 132, 652– 661.
Rassweiler, A., Costello, C., and Siegel, D. A. 2012. Marine protected areas and the value of spatially optimized fishery management. Proceedings of the National Academy of Sciences, 109: 11884 LP – 11889. http://www.pnas.org/content/109/29/11884.abstract.
Schneider, M. K., Lüscher, et al. 2014. Gains to species diversity in organically farmed fields are not propagated at the farm level. Nat. Commun. 5, 4151. doi: 10.1038/ncomms5151
Shepherd, S., Gerritsen, H.D., Kaiser, M.J., Reid, D.G., 2012. Spatial heterogeneity in fishing creates de facto refugia for endangered Celtic Sea elasmobranchs. PLoS ONE 7, e49307
Sodhi, N.S., Butler, R., Laurance, W.F.L., Gibson, L. 2011. Conservation successes at micro-, meso- and macroscales. Trends in Ecology & Evolution, 26, 11,585-594.
Stirling, D. A., Boulcott, P., Bidault, M., Gharbi, K., Scott, B. E., and Wright, P. J. 2018. Identifying the larva of the fan mussel, Atrina fragilis Pennant, 1777 Pinnidae. Journal of Molluscan Studies, 84: 247–258. https://doi.org/10.1093/mollus/eyy015.
Stirling, D. A., Boulcott, P., Scott, B. E., and Wright, P. J. 2016. Using verified species distribution models to inform the conservation of a rare marine species. Diversity and Distributions, 22: 808–822. John Wiley & Sons, Ltd. https://doi.org/10.1111/ddi.12447.
Sundblad, G., Bergström, U., Sandström, A., & Eklöv, P. 2013. Nursery habitat availability limits adult stock sizes of predatory coastal fish. ICES Journal of Marine Science, 713, 672-680.
Thomas, C. D. 2020. The development of Anthropocene biotas. Philosophical Transactions of the Royal Society B-Biological Sciences, 375.
Thomas, C. D., Franco, A. M. A., and Hill, J. K. 2006. Range retractions and extinction in the face of climate warming. Trends in Ecology & Evolution, 21: 415-416.
Tickner, D., Opperman, J. J., Abell, R., Acreman, M., Arthington, A. H., Bunn, S. E., … Young, L. 2020. Bending the curve of global freshwater biodiversity loss: An emergency recovery plan. BioScience, 70, 330– 342. https://doi.org/10.1093/biosci/biaa002
to Bühne,H.S., Tobias,J.H., Durant, S.M. et al., 2020. Improving Predictions of Climate Change–Land Use Change Interactions. Trends in Ecology & Evolution, In press. https://doi.org/10.1016/j.tree.2020.08.019
Tscharntke, T., Tylianakis, …and Westphal, C., 2012. Landscape moderation of biodiversity patterns and processes - eight hypotheses. Biological Reviews 87, 661-685.
Valdemarsen, J. W., Jørgensen, T., and Engås, A. 2007. Options to mitigate bottom habitat impact of dragged gears. Food & Agriculture Org.
von Wehrden, H., Abson, D., ..and Seppelt, R. 2014. Realigning the land-sharing/land-sparing debate to match conservation needs: considering diversity scales and land-use history. Landsc. Ecol. 29, 941–948. doi: 10.1007/s10980-014- 0038-7
Yamamuro, M., Komuro, T., Kamiya, ..and Kameda, Y., 2019. Neonicotinoids disrupt aquatic food webs and decrease fishery yields. Science 366, 620-+.
Zonneveld, I. Scope and Concepts of Landscape Ecology as an Emerging Science. In Zonneveld and Forman, 1990. Changing landscapes: an ecological perspective, Springer, NY
Disclaimer: Scottish Natural Heritage (SNH) has changed its name to NatureScot as of the 24th August 2020.
At the time of publishing, this document may still refer to Scottish Natural Heritage (SNH) and include the original branding. It may also contain broken links to the old domain.
If you have any issues accessing this document please contact us via our feedback form.



