NatureScot Research Report 1291 - Review of chough management between populations - a comparison of the biotic and abiotic factors influencing chough populations across the UK and Irish range
Year of publication: 2024
Authors: David Norfolk and Gavin Siriwardena (British Trust for Ornithology)
Cite as: Norfolk, D. and Siriwardena, G. 2024. Review of chough management between populations - a comparison of the biotic and abiotic factors influencing chough populations across the UK and Irish range. NatureScot Research Report 1291.
Keywords
red-billed chough; Pyrrhocorax pyrrhocorax; regional population trends; climate change; veterinary pharmaceuticals; habitat management; livestock grazing; food availability
Background
Chough in the British Isles are a species that feeds primarily in coastal, grazed grassland and that nest in holes in cliffs. Their nesting habit means that chough use nest boxes and quarries in some parts of their range, as well as natural sea cliffs. Other important foraging habitats, particularly at certain times of year and for specific populations, are sand dunes, mown silage fields, moorland, ploughed fields, stubbles and the tideline (Coombs, 1978). The diet consists almost entirely of invertebrates, access to which requires a short sward, although both probing in the soil (for earthworms, fly and moth larvae) and pecking on the surface (for ants, spiders and beetles) are used (Coombs, 1978). Chough largely feed in flocks during the non-breeding season and remain in (long-term bonded) pairs for breeding, but have a fluid social structure and are not strongly territorial, except close to the nest site (Coombs, 1978). Observational studies suggest that unpaired birds may help breeding pairs to raise chicks in some circumstances (Cowdy in Coombs, 1978), as is known for other corvid species (e.g., Richner, 1990). On Islay, where many birds are individually colour-ringed, several cases have been recorded of two siblings breeding with an unrelated partner in a trio (E. Bignal, pers. comm., 2023).
Scotland’s chough are at the northern-most edge of the species’ global geographical range. In Scotland, as elsewhere in Britain, chough have shown a protracted decline and range contraction over perhaps 200 years (Coombs, 1978). They are now restricted to the islands of Islay and Colonsay, but an increasing risk of regional extinction within the next 50 years has been identified due to the critically small population size (Trask et al., 2020; Stanbury et al., 2021). Since 2010, a programme of emergency supplementary feeding and parasite treatment, delivered by the Scottish Chough Study Group under contract to NatureScot, has stabilised the population, but further conservation action is needed to enhance their natural food supply and to address genetic threats, in order to develop a long-term solution.
The abundance and demography of chough on Islay have been monitored intensively since 1981, allowing rigorous population viability analyses to reveal that the mechanism for the recent population decline has been reduced first-year survival (from fledging to age one year, Reid et al., 2011). This has been attributed to reduced food sources in flock feeding sites (Reid et al., 2009), combined with high parasite burdens (Trask et al., 2020) and a genetically-driven recessive blindness condition that is expressed phenotypically due to the impoverished gene pool in the relict population (Trask et al., 2016). However, it remains possible that additional factors may also be involved, and the exact interactions between land management and food availability could be better understood. Nevertheless, the short-term (to date) success of supplementary feeding suggests that some food supply effect has been important.
Supplementary feeding has been conducted on Islay since 2010. Trask et al. (2020) report that c.82% of colour-ringed sub-adult and adult chough in the population that were known to be alive used the supplementary food between 2010 and 2018. The annual survival rates of all three age-classes (first-year, second-year and adult) were found to be positively associated with indices of use of supplementary food, while a greater proportion of breeding attempts were also successful in fed than in unfed areas. Research is continuing, but these results are strongly suggestive of acute food-limitation in the contemporary Islay population.
In addition, Trask et al. (2020) report that 58% of chough found dead on Islay that were examined from 2004-2018 (n = 14/24) had pathologically significant parasite burdens, potentially contributing to their mortality and low survival across the population. Parasites could operate as a proximate cause of death in birds that are weakened by lack of food or genetic deficiencies. Treatment of first-year chough showing symptoms of parasite infection with antihelminthic drugs increased their survival.
The results from population viability analyses by Trask et al. (2020) indicated that supplementary feeding (with associated parasite treatments) is now essential to maintain the population, but ultimately insufficient, as inbreeding will increase. Therefore, long-term conservation management in Scotland is likely to require a combination of management of food resources and genetic reinforcement, but continuing supplementary feeding and parasite treatment is critical in the short term.
The development of management approaches for chough on Islay, in particular, has been focused on the specific situation on the island due to conservation urgency and the possibility of high specificity given the policy focus on a small geographical area, leading to highly targeted potential solutions (e.g. Reid et al., 2009). This must be a positive, given that a comprehensive understanding of the ecology of the system underlies the focus, but it is also possible that ecological evidence relating to potential solutions from other parts of the chough’s range can also inform what conservation measures will be effective in Scotland. Therefore, it will be valuable to evaluate those that have been used for comparable populations elsewhere, with respect to the similarities and differences in drivers of change and ecological context. Specifically, direct supplementary feeding is not sustainable in the long-term and needs to be replaced with the provision of the equivalent ecological resources from habitat management and agricultural practices, together with effective enhancement of the gene pool. Both management and translocation measures from elsewhere may therefore provide valuable lessons for the Scottish situation, but the context of chronic, long-term decline and extreme range contraction must also be taken into account. In particular, the establishment of potential stepping stone populations between Islay and the nearest chough populations in Ireland and the Isle of Man needs to be considered to ensure long-term genetic viability within a larger metapopulation. This report aims to collate the evidence for the status, drivers of change and management effects concerning the Scottish and other relevant chough populations.
Main findings
- The Scottish chough population is of high conservation concern, despite ongoing interventions that allow it to persist. Without this active intervention it is very likely to go extinct, so maintenance of supplementary feeding and parasite treatments are critical in the short term. Long-term population sustainability will require sustainable habitat management and/or agricultural practices that provide sufficient food resources all year round, combined with genetic reinforcement of the population.
- Although there are indications that breeding success is somewhat higher in healthier populations, strong demographic comparisons between regions are not currently straightforward because results are not reported in a standard way. Formal inter-regional analyses would be valuable and could increase understanding of the drivers of population change significantly, but require novel integration of the data from diverse local groups.
- A wide range of biotic and abiotic factors could affect chough populations, and there are no obvious factors that are likely to be critical for the Scottish birds that have not been investigated. There are also no clear factors that affect the Scottish population uniquely, but comparatively healthy populations have not been studied in the same depth, which is a gap in our knowledge of the species.
- Conservation management measures applied for chough across the British Isles have many common factors and mostly involve the provision of foraging habitat, based on widely accepted key factors for foraging.
- There have been few strong evaluations of the effects of management interventions aimed at benefiting chough (as opposed to measuring environmental effects on their ecology). This means that there is no direct evidence for the efficacy of specific possible management approaches. Some novel intervention concepts have promise, but need to be trialled in respect of their effectiveness in practice.
- Based on the current understanding of chough habitat requirements, the available management approaches for grazing and livestock management practices should deliver suitable foraging habitat for chough. However, the details of the ecology supporting food resource availability and its relationships with management could be understood better, as where habitat may appear suitable, prey species may still be limited due to past or current land management. Inter-regional comparisons between the Scottish chough context and those of other populations would be valuable.
- Enhancing the genetic diversity of the Scottish population is essential for its survival, but will be challenging. There is no guarantee that captive bird releases will succeed in adding to the gene pool, for example released birds may not interbreed with the existing population (there is evidence that this was the case in Cornwall), and release protocols need to be developed in consultation with the other territories where releases have been successful and unsuccessful. Broader scale habitat management and possibly other release sites should also be considered to facilitate the operation of a metapopulation, in the form of a stepping-stone population along the west coast of the UK.
Acknowledgements
This review was funded by NatureScot. A number of individuals and organisations have provided crucial information for this document that would not be otherwise accessible via conventional literature searches. We would particularly like to thank the following for their communication and offers of help:
Ireland: Sinéad Cummins, National Parks and Wildlife Service
Scotland: David Jardine; Eric Bignal, Scottish Chough Forum; Ellen Bird, Morven Laurie, Dave Parish, NatureScot.
Isle of Man: Neil Morris, Manx Birdlife.
Northern Ireland: Adam McClure, BTO Regional Rep, Co. Antrim.
Wales: Julian Hughes, RSPB Cymru; Ian Johnstone, RSPB; Jane Hodges and Bob Haycock; Sarah Mellor, Pembrokeshire Coast National Park Authority; Tony Cross and Adrienne Stratford; Patrick Lindley.
England: Hilary Mitchell, Cornwall Bird Watching and Preservation Society; Ray Hales, Paradise Park Wildlife Sanctuary, Cornwall.
John Calladine, Ian Johnstone, Dave Parish, Morven Laurie, Sinéad Cummins, Ellen Bird and Jen Graham provided helpful comments to improve previous drafts of this report.
1. Introduction
The red-billed chough Pyrrhocorax pyrrhocorax (hereafter chough) is a crow (Corvidae) species with a geographical range extending from the western coasts of Britain and Ireland, east through southern Europe, North Africa, Central and East Asia. It is a species of European Conservation Concern (Annex 1, EC Birds Directive). Chough in the UK and Ireland (and Brittany) are recognised as the distinct sub-species pyrrhocorax (Cramp et al., 1994) and are assigned Schedule 1 species status (Wildlife and Countryside Act, 1981). This reflects ecological and biogeographical differences from the bulk of the range: birds in the British Isles and northwest France are on the edge of the range and live in coastal habitats, while the rest of the distribution is found in upland, inland regions. Population data are highly biased towards Europe, but Europe accounts for only c. 5% of the global population. Long-term records of decreases in European populations (Madge and Burn, 1993) lead to an estimation of the overall trend by the IUCN to be ‘declining’, but globally the species is also of ‘Least Concern’, reflecting the wide distribution of the majority of the population from Asia Minor to east Asia (BirdLife International, 2016). Given the geographical and ecological differences, it would seem unwise to reach conclusions about the chough status in the British Isles from evidence from the rest of the range. High conservation importance may not be justifiable in terms of the global population and current classification at the species level, but the pyrrhocorax sub-species is arguably sufficiently distinct, particularly in respect of the national and regional fauna, to support conservation priority. Chough may also be a figurehead species for the conservation of low-intensity agricultural ecosystems and the mosaic of habitats that such systems generally provide (Reid et al., 2009), although its potential to do this in practice in a UK context must be limited by its coastal-only distribution.
Chough were Amber-listed (UK) in 2009 (Eaton et al., 2009), due to being categorised as a Species of European Conservation Concern, but this criterion was removed in the 2015 UK assessment and the species remains on the Green List as of 2021, although the species is listed as Vulnerable in the IUCN2 UK assessment (Stanbury et al., 2021). ‘Green’ status reflects an overall lack of pronounced population change during recent decades and the period for which we have monitoring data, but declines are believed to have occurred over the preceding 150 years or so, leaving the extant, remnant populations in northwest Europe (Robinson, 2005). However, the overall current status masks risks of regional extinction of isolated populations within Britain and Ireland, in particular, and the species’ vulnerability is higher when these populations are considered individually. Regional differences in chough population trends are apparent: increases (or stability) in Cornwall and south Wales, but declines in Scotland and north and mid-Wales, as well as a local extinction in Northern Ireland. In Ireland, results from a chough census which took place in 2021 show localised declines in some regions, but stability overall (Colhourn et al., 2023), and, on the Isle of Man, recent anecdotal evidence suggests a perceived decline in a previously stable population, with Manx BirdLife planning an island-wide chough census in 2023 to investigate these observations (N. Morris pers. comm., 2022). Understanding the factors behind these differences in population trends and responses to previous and current management efforts should inform conservation actions in Scotland and elsewhere and facilitate informed policies and interventions.
1.1 Purpose of this review
The purpose of this review is to collate and synthesise all relevant literature and summary data on chough population dynamics, population threats and conservation management evidence that is currently available. By gathering information from key chough researchers and conservationists, we aimed to compile both published and unpublished literature on factors affecting chough populations from across the UK and Irish range. It is hoped the assimilation of this information will provide a greater understanding of factors influencing the chough populations across their respective regions, which will help to inform future conservation measures for Scottish birds and other populations within the wider British Isles, by identifying influences that both affect only individual regional populations and are common across multiple populations.
NatureScot, supported by the Scottish Chough Forum, are currently scoping a long-term programme of recovery for chough in Scotland. The results of this review are intended to collate the evidence for factors influencing chough populations across the British Isles, in order to inform conservation actions in Scotland and elsewhere, and to support the possible development of a wider recovery programme, including facilitating exchanges and linkages between otherwise isolated populations. There has been a recent reintroduction programme in Jersey, which is considered in the relevant section, but otherwise, the focus of the review is on birds in Britain and Ireland.
2. Methods
The review was divided into four sections, with rationales as described in sections 2.1-2.4. The specific methods applied for each review are then detailed in section 2.5.
2.1 Integrated review of chough population trends and dynamics
Differences in the temporal trends shown by populations in different regions, where they are subject to different environmental influences, can be informative about the factors to which a species is sensitive. Further, complementary information on population demography, i.e., dynamics, can indicate the life history stage where critical pressures have acted. This approach was central to the diagnosis of causes of decline among farmland birds in the UK (Siriwardena et al., 2000), for example. However, differences in population context and ecology between regions (such as the relative occurrence of immigration and the features of habitats that are available) could limit the extent to which comparisons are informative. For example, a larger, more connected population may be intrinsically more stable than more isolated populations. It is, therefore, important to consider population dynamics evidence with care.
Collating evidence about chough in Britain and Ireland is challenging, because of the species’ rarity and the fragmented nature of the wider population: reliable, standardised data are not collected by national monitoring schemes and small, regional populations tend to be difficult to study, in terms of generating sufficient sample sizes for robust analyses. Further, if regional populations differ ecologically, pooling data across different regions may be inappropriate. Hence, evidence is likely largely to be found in locally published sources for each region, having been collected using locally specific methods, and possibly may be difficult to access and to standardise.
2.2 Review of the evidence for the influences of biotic and abiotic factors (and interactions between them) on chough population dynamics
Species’ populations can be influenced by a wide range of environmental influences, both ecological (biotic) and related to the physical environment (abiotic). The key abiotic factors influencing chough could include climatic conditions, seasonal weather conditions, nest site availability (quarries, crevices in sea cliffs and out buildings), soil type and health (acidity, nitrogen and phosphorus levels), and underlying geology. Biotic factors could include sward structure and the effects of grazing of wild and domestic mammals (notably rabbits), avermectin or other veterinary pharmaceutical contaminants, soil biodiversity and abundance (i.e. food availability) and its drivers, and interactions with other wild species (including disease, competition and predation). Further, interactions between these factors may also be critical in terms of when and how they impact upon chough, because some influences may only be significant, for example, when conditions are harsh. It will also be important to consider the demographic mechanisms by which these influences may affect chough abundance, as this will affect how interventions might best be targeted. For example, winter habitat or weather conditions would be most likely to primarily affect overwinter survival, whereas nest site availability would affect breeding success.
2.3 Review of population establishment and conservation management measures
The status of British and Irish chough as vulnerable means that a range of management approaches have been taken to promote the species, but these vary with respect to the context of local populations and the threats that have been identified. A key contrast, or variation, may be that some local populations are remnants and/or declining, suggesting that aspects of the local environment have become unsuitable, while others are newly colonising or spreading, wherein management potentially involves enhancing nearby areas to allow further spread. In both cases, the approaches that have been successful in other locations may also be effective in Scotland, while unsuccessful approaches may provide important lessons, although population context must also be taken into account.
A similar approach to that used for population dynamics was undertaken to collate information regarding management approaches for chough, for the same reasons. The evidence found included that from local bird reports and atlases and that from consultation with local experts in each region, revealing the range of conservation management that has been applied to support existing populations and the establishment of new ones (via natural colonisation or reintroduction). Similarly, information on how and why some populations have been lost (e.g. from Northern Ireland in the 2010s, from southeast Scotland in the later 20th Century and from southwest England in the early 20th Century), or have seen only intermittent, short-term establishment (e.g. Mull, Galloway) was also sought. Relevant management measures in the last two decades were mostly implemented via agri-environment schemes (AESs), so scheme handbooks were consulted.
2.4 Review of evidence for the effects of management interventions on chough abundance and population dynamics
Clearly, evidence for the effectiveness of interventions should be an important factor in determining whether their wider use is to be recommended. Once again, the relevance of the evidence will depend on context, but could come from a range of sources. Interventions will notably include specific grazing and mowing measures that are designed to enhance habitat for chough (such as the Scottish Government’s Agri-Environment and Climate Scheme (AECS) ‘Chough Grazing Management’ option and ‘Chough Mown Grassland’ option). It will be important to consider both core intervention types, as might be tested in field trials or based on empirical evidence, and the AES options that codify them, because efficacy can be reduced following implementation in practice. Evidence could come from measured, hypothetical (i.e. based on logical deduction from ecological knowledge and principles) or modelled effects on chough numbers or demographic rates, notably breeding success and survival. Evidence quality could be revealed by factors including sample size, study duration, statistical significance, qualitative versus quantitative results, presence of confounding factors, and temporal and geographical relevance to the Scottish context. In addition, how well the sampling of chough parameters has been matched to the management activity could be critical; for example, the certainty with which habitat management can be associated with particular pairs whose breeding success is measured and the extent to which multiple interventions have been applied together, limiting the potential to assess their independent effects.
2.5 Literature review approach
For each individual review, we used a combination of systematic and traditional review protocols, focusing on the British Isles subspecies of chough in the UK and Ireland. Both peer reviewed and conservation/land management reports were searched to provide information. Utilising extensive access to online material via Google Scholar and Web of Science, academic search engines, and the resources of the Cambridge University Library, we accessed relevant resources that were available electronically, and carried out the searches using a comprehensive list of search terms based on key literature. Search results were refined by sifting manually to remove duplicate sources and by following promising references within the publications that were identified in the first instance. Harzing’s Publish or Perish© software was used to filter published literature that was less than 30 years old, where more up-to-date information was required for certain age-relevant topics (e.g. population assessments and the use of veterinary pharmaceuticals on livestock).
It was evident in the preliminary stages of the literature search that information pertaining to red-billed chough literature was limited, so search terms were generally restricted to using only two words (including ‘chough’).
Individual searches made in combination with ‘chough’ as the primary search term:
- AND agri-environment OR farmland OR management OR conservation OR population OR UK OR disease OR parasites OR measures OR prey OR livestock OR land-use OR predation OR climate OR distribution OR trend OR genetics OR foraging OR reproductive OR demographics OR nesting OR survival OR food OR ecology OR effects OR dung OR insects OR invertebrates OR avermectin OR drugs OR pharmaceuticals OR veterinary OR tourism OR disturbance OR grazing OR weather.
Additional searches were made that may not have been directly related to chough ecology, but could provide relevant information to support the evidence, or benefit discussion throughout this review:
- avermectin OR anti-parasitic AND birds OR livestock OR dung OR soil OR insects OR invertebrates.
- birds AND grazing AND sward OR livestock OR dung OR invertebrates.
In addition, since much relevant information was likely to be obscure and/or unpublished, we (a) used the BTO’s library (one of the two most comprehensive ornithological libraries in the UK) to search for relevant articles in local and regional bird reports and journals from across the UK and Ireland, and (b) utilised our network of contacts to approach the key chough researchers in Wales, Scotland, England, the Republic of Ireland and the Isle of Man, who supplied us with the most recent population and ecological results.
3. Population trends and breeding success
First, we summarise the current distribution and regional breeding population sizes of chough across the British Isles, then provide detail on the population trends and breeding success by region.
3.1 Populations of breeding chough in the UK and Ireland
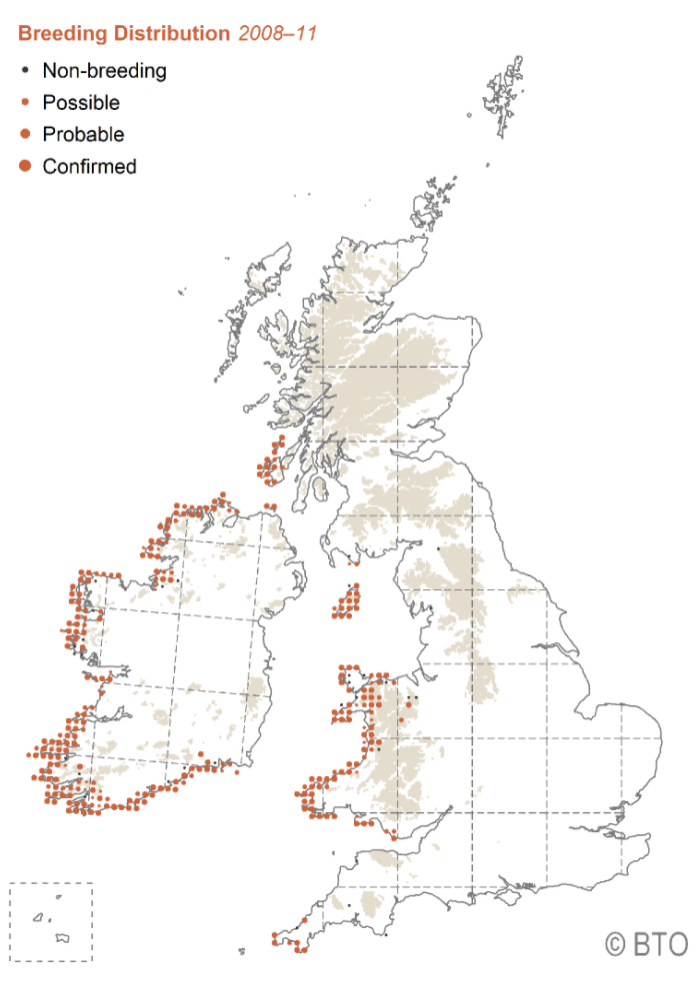
Map of the UK and Ireland showing the breeding distribution of chough during the period 2008 to 2011, from the BTO Breeding Bird Atlas. Orange dots show possible, probable and confirmed breeding locations via the increasing size of dots. All breeding chough are found on the western seaboards, with most birds in western Ireland, some in western Wales and the Isle of Man, and relatively few on Islay, Colonsay and Oronsay in Scotland.
Chough are predominantly distributed across the western coastlines of the UK and the Republic of Ireland (hereafter Ireland), in several well-defined but fragmented populations (Balmer et al., 2013). Ireland currently holds the largest population in the region and supports more than 70 % of the Northwest European population (Birdlife International/EBCC, 2000), with around 390 confirmed/probable breeding pairs (Colhoun et al., 2023). In 2014-15, Wales supported the largest percentage of UK birds (55%), with 236 breeding pairs, followed by the Isle of Man (31%) with 133 breeding pairs. Scotland’s population, at the time these figures were collated, comprised 11% of the total: a population of fewer than 60 pairs persisting on the islands of Islay and Colonsay, although with evidence of an ongoing decline. However, these results were obtained from 2014/15 data following the last UK and Isle of Man population assessment (Hayhow et al., 2018) and the detailed proportions are therefore expected to have changed by now. All populations are known to have seen both regional and local population changes since the 2014/15 population assessment. During the last national census, mainland England (Cornwall) supported only seven breeding pairs (2% of the UK population), which had tripled by 2021 (CBWPS, 2021). A smaller population has now been breeding in the wild on Jersey since 2015, following a captive breeding and reintroduction programme on the island. In 2021, the Jersey population was around 30 birds (Corry et al., 2021). Chough have not bred in Northern Ireland since the one remaining breeding pair disappeared from Rathlin Island in 2017 (Allen and Mellon, 2021).
Historical evidence suggests that chough were much more widespread than they are today (Yalden and Albarella, 2009), with declines being observed in the nineteenth Century in Wales, England and the Isle of Man, and a long-term range contraction in Scotland. However, there is evidence of recovery in some areas in the early part of the twentieth century (Owen, 1989). The declines have been attributed to a reduction of foraging habitat through changes in land-use and farming intensification (Bullock et al., 1983). From the 1960s onwards, there have been a number of censuses conducted on breeding chough in the UK and Ireland, which have provided more accurate population estimates. There have been four national censuses in the UK and Isle of Man: in 1982 (Bullock et al., 1983), in 1992 (Green and Williams, 1992), in 2002 (Johnstone et al., 2007) and in 2014 (Hayhow et al., 2018), and two surveys confined to Scotland, one in 1986 (Monaghan et al., 1989) and one in 1998 (Cook et al., 2001). There have been five censuses in Ireland (Cabot 1965; Bullock et al., 1983; Berrow et al., 1993; Gray et al., 2003; Colhoun et al., 2023). Section: 3.2 Regional population trends and breeding success below summarises the results of these censuses.
3.2 Regional population trends and breeding success
The available data on population size and change are summarised in Tables 1-3. Based on the available data, overall chough numbers appear to have remained relatively stable across the region in the last 30 years, apart from in Scotland, north and mid-Wales. In Northern Ireland, the very small population has undergone a gradual downward trend between survey periods, resulting in extirpation (Balmer et al., 2013). Available estimates of breeding success are summarised in Table 4; note that standard information is not available for all regions and the figures that have been quoted, or were available for collation here, are not necessarily equivalent. This is particularly true for the treatment of numbers of pairs or sites, in particular unsuccessful pairs or non-productive nest sites. An important parameter for demography is breeding success per pair, i.e., including zero values for pairs for whom nesting attempts failed or that did not breed. However, it was frequently unclear in the available sources whether all of these zero values were included in calculations, and they were clearly sometimes excluded. Hence, results are presented for the average numbers of fledglings per nest both including and excluding zeroes, where possible, or for the one of these that was available; the values including zeroes should be treated with particular caution as there could be hidden variations in the treatment of zeroes between data sources. Note also that differences in field methods could have a significant impact on the productivity estimates that are presented in Table 4: they depend on how occupied sites or pairs are defined (e.g. ‘possible’ and ‘probable’ breeding) and counting fledged (possibly hiding) chicks can be problematic, especially if families have left the immediate vicinity of the nest site. A formal comparison of regions really needs standardisation of all of these aspects of the methods (e.g. Hayhow et al., 2018); otherwise, it is likely that there will be significant biases.
Table 1. Summary of chough population estimates (breeding pairs) for UK, Isle of Man and Ireland from the past 30 years from national censuses.
‘NI’ denotes no information available.
|
Country/region |
1992 |
1998 |
2002/03 |
2014 |
2021 |
% change 1992 - 2021 |
|---|---|---|---|---|---|---|
|
England* |
0 |
0 |
0 |
7 |
23 |
+2300 |
|
Isle of Man |
68 |
78 |
128 |
133 |
NI |
NI |
|
Scotland |
82 |
58 |
71 |
53 |
NI |
NI |
|
Wales |
151 |
173 |
228 |
236 |
NI |
NI |
|
Northern Ireland |
2 |
1 |
1 |
1 |
0 |
-200 |
|
UK and Isle of Man |
303 |
310 |
429 |
433 |
NI |
NI |
|
Ireland |
428 |
NI |
445 |
NI |
386 |
-10 |
*Data derived from continuous annual monitoring
UK and Isle of Man estimates (Hayhow, 2018); Ireland estimates (Gray, 2003)
Table 2. Breakdown of population estimates of breeding pairs per county/region for Ireland. Pairs are confirmed and probable breeding pairs based on two standard visits.
|
Country |
County |
1992 survey |
2002/03 survey |
2021 survey |
% change 1992-2002/3 |
% change 2002/3-2021 |
|---|---|---|---|---|---|---|
|
Ireland |
Wexford |
8 |
0 |
4 |
-800 |
+400 |
|
- |
Waterford |
18 |
11 |
25 |
-38 |
+127 |
|
- |
Cork |
153 |
134 |
173 |
-9 |
+29 |
|
- |
Kerry |
112 |
141 |
68 |
-15 |
-52 |
|
- |
Clare |
19 |
11 |
31 |
-14 |
+182 |
|
- |
Galway |
26 |
16 |
14 |
-47 |
-13 |
|
- |
Mayo |
34 |
43 |
31 |
+23 |
-28 |
|
- |
Sligo |
10 |
7 |
3 |
-14 |
-57 |
|
- |
Leitrim |
1 |
4 |
1 |
+75 |
-75 |
|
- |
Donegal |
47 |
78 |
44 |
+28 |
-44 |
|
- |
Total |
428 |
445 |
394 |
-4 |
-11 |
(Adapted from Colhoun et al, 2023)
Table 3. Breakdown of population estimates of breeding pairs per county/region for UK and Isle of Man.
Pairs are confirmed and probable breeding pairs based on two visits (1992) and two visits plus all other information (2002, 2014, 2021). Results for England are derived from annual nest monitoring data of successful breeding pairs and not the standardised ‘two visit’ method as with all other regions. Percentage change of breeding pairs in UK and Isle of Man between surveys 1992, 2002, 2014, and in England and in 2021. ‘NI’ denotes no information available.
|
Country |
County |
1992 |
2002 |
2014 |
2021 |
% change 1992-2014 |
% change 2002-2014 |
% change 2014-2021 |
|---|---|---|---|---|---|---|---|---|
|
Scotland total |
- |
82 |
71 |
58 |
NI |
-29 |
-18 |
NI |
|
- |
Islay |
69 |
56 |
48 |
NI |
-30 |
-14 |
NI |
|
- |
Colonsay |
9 |
14 |
10 |
NI |
+11 |
-40 |
NI |
|
- |
Jura |
2 |
0 |
0 |
NI |
-100 |
0 |
NI |
|
- |
Dumfries and Galloway |
1 |
1 |
0 |
NI |
-100 |
-100 |
NI |
|
- |
Mull |
1 |
0 |
0 |
NI |
-100 |
0 |
NI |
|
Wales total |
- |
150 |
228 |
236 |
NI |
+57 |
+3 |
NI |
|
- |
Anglesey |
13 |
39 |
40 |
NI |
+207 |
+3 |
NI |
|
- |
Caernarfon |
56 |
84 |
87 |
NI |
+55 |
+4 |
NI |
|
- |
Denbighshire |
0 |
2 |
2 |
NI |
Increase |
0 |
NI |
|
- |
Meirionnydd |
8 |
20 |
18 |
NI |
+125 |
-10 |
NI |
|
- |
Montgomery |
4 |
1 |
0 |
NI |
-100 |
-100 |
NI |
|
- |
Ceredigion |
15 |
25 |
27 |
NI |
+80 |
+8 |
NI |
|
- |
Pembrokeshire |
53 |
59 |
58 |
NI |
+9 |
-2 |
NI |
|
- |
Glamorgan |
1 |
3 |
4 |
NI |
+300 |
33 |
NI |
|
England |
Cornwall |
0 |
1 |
7 |
23 |
Increase |
+600 |
229 |
|
Northern Ireland |
Antrim (Rathlin Island) |
2 |
1 |
1 |
0 |
-50 |
0 |
0 |
|
Isle of Man 2014/15 |
- |
68 |
128 |
133 |
NI |
+94 |
+16 |
NI |
|
UK and Isle of Man total |
- |
302 |
429 |
433 |
NI |
+34 |
+3 |
NI |
(Adapted from Hayhow, 2018)
Table 4. Summary of average breeding success in regional populations.
Fledglings/pair are calculated for all available records, for the periods stated, including zeroes for unsuccessful pairs or reporting just from successful nests, depending on what was available or interpretable from data sources. Calculations were simple totals, ratios and/or averages from the available figures, depending on what has been published, attempting to convert all into the same scale. However, details of how ‘pairs’ were defined were not available for many datasets and probably varied (see main text), meaning that it would be unwise to interpret regional differences in detail. Some calculations used annual totals of chicks and nests, and some annual records of productivity, which in turn could have been reported per nest or per local population. Figures may not match those in other publications because calculations have been done differently (if in doubt, take values from the primary sources, not here). Ranges reflect summaries of minimum and maximum annual values across years. Averages across years are weighted by the sample sizes in each year (so, for example, the Cornwall data are dominated by the latter years in the time series). Standard errors are provided where they could be estimated from the data that were available, derived from whole samples or, mostly, from annual averages; they should therefore be treated with caution as they represent under-estimates of the true variation in most cases.
|
Region |
Years measured |
Total no. of nests |
No. successful (No. of broods) |
Fledglings per pair (SE) Including zeroes |
Fledglings per pair (SE) Excluding zeroes |
Source |
|---|---|---|---|---|---|---|
|
Scotland - Islay |
2010-2018 |
538 |
287 |
1.29 (?) |
2.41 (?) |
Trask et al. (2020) |
|
Scotland - Colonsay |
1990-2018 |
289 |
- |
2.00 (?) |
- |
Jardine et al. (2019) |
|
Isle of Man |
2002-2016 |
459 |
401 |
1.95 (0.35) |
2.24 (0.37) |
Moore (2006-2020) |
|
Cornwall |
2002-2021 |
204 |
112 |
2.10 (1.27) |
3.08 (0.37) |
CBWPS (2021) |
|
Ireland* |
2002-2003 |
- |
98 |
- |
2.43 (1.23) |
Gray et al., (2003) |
|
Wales - Gower |
1992-2020 |
73 |
- |
1.62 (?) |
2.51 (0.41) |
Hodges and Haycock (2020) |
|
Wales - Pembrokeshire |
1992-2020 |
1675 |
- |
1.64 (?) |
2.54 (0.28) |
Hodges and Haycock (2020) |
|
Wales - north and mid-west |
2011-2020 |
1666-1831 |
680-845 |
- |
2.96 (0.07) |
Cross and Stratford chough project |
* Additional breeding success information is reported in Boylan, M. (2011) The ecology of the Chough in south west Ireland. PhD Thesis, National University of Ireland, Cork (S. Cummins, pers. comm. 2023). However, the thesis is not published and was not available at the time of writing. The summary data of which we are aware are not in a format that allows incorporation or conversion to match the figures in this table, but this might perhaps be possible with access to the thesis.
3.2.1 Scotland
There is evidence to suggest that chough populations have had a long history of decline and recolonization throughout much of Scotland, (e.g. records as far south as the borders (1867) and as far north as Orkney during the Iron Age) (Forrester et al., 2007). Between the 1963 and 1992 censuses, the Scottish population had increased from 11 breeding pairs in 1963 (Rolfe, 1996) to 88 pairs by 1988 (Monaghan et al., 1989). However, the population then declined from the 1992 census period onward, while all other populations, until recently, (except for the very small Northern Irish population) were undergoing population increases. As a result, the breeding range of Scottish chough retracted to just three islands (Islay, Colonsay and Oronsay) within this period, having recolonised these islands in the mid-1960s, with the last breeding occurrence prior to this in the early 1900s (Thom, 2010). They also previously bred on the islands of Jura and Mull, and on the mainland in Dumfries and Galloway at the time of the 1992 census, and also on Kintyre from 1964-83. Since the last census in 2014, numbers are believed to have stabilised to some degree due to supplementary feeding and successful treatment of parasites (Trask et al., 2020), but the population continues to remain particularly vulnerable, due to its restricted breeding range, and recruitment of young birds into the breeding population remains low. Islay remains their stronghold, supporting a relatively stable population of c.50 breeding pairs since 2000, which constitutes c.80% of the Scottish population (Monaghan et al., 1989; Finney and Jardine, 2003). The Colonsay and Oronsay population densities have remained relatively low compared to Islay, with a peak breeding population of between 15 and 20 breeding pairs between 2000 and 2010, but have seen a gradual decline since then (Jardine et al., 2019), down to 3-4 pairs in 2023 (D. Jardine, pers. comm., 2023).
Fledging success data were obtained from the Annexes to Trask et al. (2020) and a Scottish Chough Study Group submission to the 2020 Scottish Chough Forum (M. Laurie, pers. comm., 2022). In 1998, an average of 2.07 (± 0.19 se, n=43) fledglings per breeding pair were produced on Islay and 2.78 (±0.40 se, n=9) on Colonsay. No significant differences in fledgling success between these two islands was evident, while Jura and Galloway only had a single breeding pair, producing two and four fledglings, respectively, and a single breeding pair on Mull did not produce young (Cook et al., 2001). In the long-term data, there was some evidence that breeding success has been higher on Colonsay than on Islay in recent years, but differences are marginal and potentially influenced by differences in the definition of zero success nests (Table 4, Table A1 and Table A2). In general, however, breeding success in Scotland appears somewhat lower than in some other regions (albeit with the same caveat about data interpretation). In Scotland, chough are a qualifying species in Special Protection Areas (SPA), listed in Annex I of the Birds Directive.
3.2.2 Ireland
The overall trend for Ireland is considered to be one of relative stability with fluctuating breeding numbers either side of the 2002/3 census, , with a reported, small, overall breeding population decrease of 10% between the 1992 and 2021 censuses. However, most recently, there have been notable gains and losses recorded at the county/local level, with a suggestion of a north-south divide, with the exception of Kerry. The historical stronghold of Cork has experienced a recovery following a decrease in 2002/3 census and has increased by 29%. In contrast the neighbouring county of Kerry (the second largest historical stronghold), has undergone a significant decrease of 52%. Conversely, Clare to the north has seen 182% and 63% increases, respectively, from the last two census periods. Waterford and Wexford, situated on the south coast, have also reported gains since 2002/3, 127% in Waterford and from zero to four breeding pairs in Wexford, such that numbers are now similar to those in 1992. The overall trend appears to be one of decrease north of Clare and up to Donegal. Although having historically smaller population sizes, Galway, Mayo, and Sligo have all undergone continued small declines from the previous two censuses. Donegal (the northernmost county) has seen a 44% reduction in numbers since 2002/3 and the highest range losses in terms of birds occupying 10-km survey squares from the 2021 census, compared with the Breeding Bird Atlas (2007-11) distribution, such that numbers are now similar to those in 1992. Leitrim has retained a single breeding pair, following an increase of three breeding pairs between the previous two censuses (Colhoun et al., 2023).
In recent decades, chough have successfully recolonised and established breeding populations on the British mainland – first on the Gower peninsular, in south Wales from 2000, and then in Cornwall in 2002, for which there is evidence to suggest they originated from Ireland (Wenzel et al., 2012). This significant range expansion, in parallel with stable breeding numbers, suggests that the Irish chough population was in relatively good health during this time period. During the 2002-03 census the average number of fledglings per successful pair was 2.43 (±1.23 se, n=98) (Gray et al., 2003). This value is not one of the higher ones among regions (Table 4), but has a large standard error, indicating high variability.
3.2.3 Wales
Wales holds around 75% of the UK chough breeding population. Available data shows an overall upward trend in Wales from the last three censuses, although only a relatively small increase over the period 2002-2014. Likewise, the historical trend from 1963 onwards has been positive for all census periods. However, there is strong variation in regional trends with declines in some areas and particular concern about falling numbers of chough breeding inland in north and mid-Wales (Cross et al., 2020; Haycock et al., 2021). Caernarfon continues to retain the largest population with a 55% increase between 1982 and 2014, with a suggestion of a levelling-off in recent years. Pembrokeshire has remained the most stable population out of all the Welsh counties, varying between 50-60 pairs between 1992 and 2014. Out of those counties that had double-figure breeding pair numbers recorded from the 1992 census, the population on Anglesey has undergone the sharpest increase, with 40 breeding pairs in 2014, representing a +207% change, and has remained stable since (Cross et al., 2020). The counties of Ceredigion, Glamorgan, Meirionnydd and Denbighshire have seen their populations remain broadly stable throughout all periods. However, Montgomeryshire had lost its last remaining breeding pair between the 2002 and 2014 census periods (Table 3).
In Pembrokeshire, the chough population has been monitored since the early 1980s (Table 3, A4). The main focus of this work is on distribution, demography and productivity, in line with the Chough Conservation Strategy for Pembrokeshire (Hodges, 1994). A particular focus of this monitoring of the breeding population within the National Park is on the islands of Skomer and Skokholm, and the Seas off Pembrokeshire SPA. Similar work is carried out in the Ramsey and St David’s Peninsula Coast SPA and the Castlemartin Cliffs SPA. These three Pembrokeshire sites are part of the Natura 2000 network of Special Areas of Conservation (SACs) and SPAs for which chough are a qualifying species. Annual nest monitoring has taken place since 1992 (Table A4; see Haycock et al., 2021 for detailed reporting). The most successful nesting year was in 2020 (61 of 73 recorded nests fledging 169 young), and an apparently high level of productivity compared to other regions (c. 2.5 fledglings per successful nest: Table A4). Productivity in 2020 (2.8 fledglings per breeding pair) was above the 29-year average, and the minimum number of fledged young was the highest ever recorded (Hughes, 2020). The lowest year of productivity recorded was 1993, but this was due to incomplete survey coverage in that year (Hodges and Haycock, 2020, Haycock et al., 2021). (The specific data shown graphically in Haycock et al. (2021) were not available for re-analysis, so Tables 4 and A4 are based on the figures that could be extracted from Welsh Bird Reports for the same period. The data published in the bird reports were not standardised, particularly in earlier years). Chough are also monitored comprehensively across the parts of the range in north and mid-Wales, with annual recording of more than 170 pairs in the counties of Anglesey, Caernarfon, Denbighshire, Meirionnydd, Ceredigion and Montgomeryshire (although zero abundance counts have been recorded in recent years for Montgomeryshire; A. Cross and A Stratford, pers. comm., 2022). The data collected contribute to national totals and the BTO Ringing Scheme database, and are published in Cross et al., 2020. Overall breeding success for this region appears to be high relative to other regions, at least among successful nests. However, between 1994 and 2019 the territory occupation rate declined by 72% in inland areas, and by 12% in coastal areas. This equates to a 27% decline in the breeding population of mid and north Wales. These results add detail to the recent periodic national census results that highlighted some concerning vice-county level changes. Furthermore, nest studies carried out during the ‘Red-billed Chough Research Programme’ found that adult survival in north and mid-Wales is lowest for birds hatched in inland areas and highest for birds hatched in coastal quarries. However, there was a marked decline in the survival of first breeding year adults (from 90.2% in 1993-99 to 75.1% in 2014-19). There was also a marked decline in the rate of recruitment of new breeding adults especially to inland breeding territories, which suggests survival around the time of recruitment to the breeding population as a period of potential demographic stress for the Welsh Chough population. Declining recruitment to the breeding population, increasing mortality affecting first-time breeders and declining productivity and nest success rate may collectively explain observed patterns in territory occupation rates (Cross et al., 2020).
3.2.4 Isle of Man
Available census results show that since the 1992 census, the Isle of Man has seen an overall positive trend in breeding numbers with an 88% increase between the 1992 and 2002 censuses, which had tailed-off to a slower rate of a 16% increase by the 2014 census (Table 5). The Isle of Man population has remained stable, particularly on the Calf of Man, where breeding numbers have remained identical (n=14 pairs) between the last two census periods. The most notable increase has been the re-colonisation of the northern part of the island, with six breeding pairs recorded during the last census. All regions have maintained relative stability between 1992 and 2014-15 censuses, apart from the west where there has been a degree of fluctuation (Table 5).
Table 5. Total breeding pairs from Isle of Man censuses 1992 to 2014-15.
|
Region |
1992 |
2002 |
2014-15 |
% change 1992 – 2014/15 |
|---|---|---|---|---|
|
West |
23 |
42 |
31 |
+35 |
|
Calf |
8 |
14 |
14 |
+75 |
|
South |
11 |
40 |
46 |
+264 |
|
East |
30 |
46 |
52 |
+73 |
|
Inland |
5 |
8 |
11 |
+120 |
|
North |
0 |
0 |
6 |
+600 |
|
Total main island, not including north |
69 |
136 |
140 |
+100 |
|
Total |
77 |
150 |
160 |
+30 |
(Moore, 2018)
Annual nest productivity data have been published by Moore (2004-2020), allowing fairly strong summary analyses to be conducted. These suggest that average breeding success has been lower than in some regions, but probably higher than that in the Scottish population (Table 4). There is no clear evidence of a temporal trend in breeding success among Isle of Man chough (Table A3). However, anecdotal evidence now suggests that chough may be in decline, as indicated by a discernible downward trend in maximum flock sizes observed over the last four years, and the 2021 Birds of Conservation Concern in the Isle of Man listed Chough as Amber (N. Morris pers. comm., 2022).
3.2.5 England
Historically, chough were widespread along the south and southwest coasts of England before numbers started to decline in the 1800s. The last breeding occurrence was in Cornwall in 1947 (Carter et al., 2003), until a breeding pair naturally recolonised the area in 2001 and successfully raised three young the following year. DNA analysis of feather samples showed that this pair had originated from southern Ireland (Wenzel et al., 2012).
Breeding chough in England have undergone a near-continuous population increase since the recolonization in 2001. An annual population increase has been observed, with the exception of 2017 (as a result of bad weather during the nesting period). The 2022 breeding season was another successful year for Cornish chough, and the trend is forecast to continue as there is plentiful, suitable, available habitat into which their breeding range can expand along the Cornish coast and as breeding numbers have now reached the threshold (20-30 breeding pairs) of what is considered a self-sustaining population by the RSPB (Table A5): the recent trend looks positive, with 70 fledglings raised from 25 pairs in 2022 (CBWPS, 2022). There is a suggestion of a reduction in fledgling success rate in recent years, although the sample size is too small to infer a genuine change in nest productivity at this stage. Additionally, initial breeding success was high relative to that in other regions, as is the overall mean of 3.08 chicks per nest fledged across all years and for all successful nests (Tables 4 and A5).
3.2.6 Northern Ireland
Northern Ireland had supported a single breeding pair between 1992 and 2002 (Gray et al., 2003), with the last breeding occurrence in 2017 on Rathlin Island. The pair often wintered along the mainland Antrim coastline and, although this was not confirmed, was believed to commute to Donegal in the Republic of Ireland. Any sightings of the species remain very scarce in Northern Ireland (McClure pers. comm., 2022). The breeding success of these birds was not measured, but was probably low, unless most fledglings emigrated to other populations.
4. Review of the evidence for the influences of biotic and abiotic factors (and interactions between them) on chough population dynamics
To be effective, chough conservation measures need to be designed to counter the influences of negative factors for population change and to promote or at least not to negate the influences of positive factors. The important influences could be ecological (biotic) or physical (abiotic), and could act via interactions between diverse influences, such that the likely success of management measures varies considerably between contexts. Multiple factors affecting chough populations are well documented, and it is unlikely that a single factor in isolation is influencing population dynamics for all regions where they occur (Table 6). Although chough in the British Isles are predominantly found in rocky coastal habitats, some nest further inland in quarries, derelict buildings or agricultural structures. Some have access to arable land as well as a variety of grassland types. Hence, the pressures and influences on different populations will vary regionally, but the set of factors across all regions should provide a fairly comprehensive guide to the potentially important drivers of habitat suitability and population change in any location.
Table 6. Summary of biotic and abiotic factors, their effects and consequences, and conservation measures.
All were identified in both published and grey literature; biotic and abiotic factors are as considered to be either probable or potential causes affecting chough populations by region; the conservation measures have been implemented in response to these factors.
|
- |
Factors influencing chough population |
Effects/Consequences |
Conservation measures currently implemented |
Source |
|---|---|---|---|---|
|
Scotland |
Loss of low intensity livestock grazing |
Reduced first year survival rates |
Year-round cattle grazing |
NatureScot |
|
- |
Lack of food availability and decrease in foraging opportunity |
Decrease in first year survival rates |
Supplementary feeding programme 2010 (Islay) |
NatureScot |
|
- |
Veterinary pharmaceutical practices (anthelminthic treatments) |
Decrease in dung invertebrates as food |
- |
Reid et al., 2009 |
|
- |
Mechanical vegetation topping |
Destruction of dung pats and associated insect food prey |
- |
Reid et al., 2009 |
|
- |
development and forestry activities |
- |
- |
Reid et al., 2009 |
|
- |
Inbreeding |
Low genetic diversity – reduced viability |
- |
Wenzel et al., 2012 |
|
- |
Increased tourism |
Disturbance to nesting and foraging birds |
- |
McKay, 2007, Hayhow et al., 2018) |
|
- |
Climate: Increased heavy rainfall events projected for Scotland |
Decrease in nesting success rates |
- |
NatureScot, SAC Chough Conservation Paper |
|
- |
Parasite burdens |
Clinical and sub-clinical effects on fitness in some birds. |
Treatment for parasites |
NatureScot, SAC Chough Conservation Paper |
|
- |
Climate |
Bad weather late spring effecting nesting success, summer droughts, extended cold periods reducing foraging ability. |
- |
Reid et al., 2009 |
|
England |
Human disturbance |
Decreased foraging time |
- |
Owen 1989, Kerribiriou et al., 2007. |
|
- |
Removal of livestock from cliff edge heath and grassland |
Increased scrub/vegetation height reducing foraging ability. |
Reinstated livestock grazing along the cliff edge and coastal pastures using agri-environment options. |
Cornwall Council |
|
- |
Illegal egg collecting |
Reduced nesting success. |
Round-the-clock volunteer nest surveillance since 2002 |
Cornwall Council |
|
Wales |
Afforestation on unstable bare/clay cliffs |
Increased vegetation and bracken encroachment reducing foraging opportunities. |
- |
Nature Gwynedd |
|
Gwynedd and Pembrokeshire |
Veterinary pharmaceutical practices ‘Avermectins’ administered to coastal livestock |
Decreased insect food availability in animal dung. |
- |
Nature Gwynedd and Chough Conservation Strategy for Pembrokeshire (Hodges 1994) |
|
- |
Natural predators (Peregrine) |
Disturbance to communal roosts and affecting fledgling success. |
- |
Nature Gwynedd and Chough Conservation Strategy for Pembrokeshire (Hodges 1994) |
|
Gwynedd |
Mining/quarrying activities |
Destruction or disturbance of nest sites. |
Nest site protection in working quarries during the breeding season; Safeguard potential nest sites/communal roosts from certain development or mining. |
Nature Gwynedd (Species Action Plan for Chough) |
|
- |
Recreation activities – climbing, ramblers, bird watchers, paragliders |
Disturbance to nesting and foraging birds. |
Liaison with British Mountaineering Council |
Nature Gwynedd (Species Action Plan for Chough) |
|
Pembrokeshire |
Coastal scrub encroachment |
Reduced foraging opportunities. |
Controlled winter burning of Gorse |
Pembrokeshire Coast National Park Gwarchod y Park; Conserving the Park 2003-2018, |
|
- |
Illegal egg collecting |
Reduced fledging success |
- |
Pembrokeshire Coast National Park Gwarchod y Park; Conserving the Park 2003-2018, |
|
- |
Cliff-top recreation activities |
Disturbance to foraging and disruption of social behaviour |
- |
Pembrokeshire Coast National Park Gwarchod y Park; Conserving the Park 2003-2018, |
|
- |
Climate related |
Spring storms impact foraging time. Nests get washed out. Under-weight chicks get water-logged and chilled causing death. |
- |
Chough Conservation Strategy for Pembrokeshire |
|
- |
Reduced genetic diversity |
long-term health and resilience of the population |
- |
Wenzel et al., 2012 |
|
- |
Possible predators (fox, weasel, stoat, ferret, polecat) |
Predation of eggs and chicks could occur (not quantifiable) |
- |
Chough Conservation Strategy for Pembrokeshire |
|
Northern Ireland |
Removal of grazing from grassy cliff slopes |
Loss of short well-grazed turf and bare ground |
- |
Allen and Mellon, 2021 |
|
Antrim (Rathlin Island) |
Illegal shooting (Rathlin Ireland) |
Accelerated population decline |
- |
Allen and Mellon, 2021 |
|
Antrim |
Intensification of grassland management and removal and loss of stubbles. |
Loss of winter foraging opportunities |
Local farmers have all contributed to the creation of more favourable foraging conditions through chough targeted agri-environment options. Introduction of chough ‘prescriptions’ on the Antrim Coast |
Allen and Mellon, 2021 |
|
- |
Recreational rock climbing |
Potential Nest disturbance causing reduction in nesting success |
- |
Allen and Mellon, 2021 |
|
Ireland |
Abandonment of land |
Loss of suitable foraging habitat if land is left unmanaged. |
- |
Gray et al., 2003 |
|
Cork (Dursey Island) |
Tourism/development |
Disturbance to foraging and nesting from proposed cable car from Irish mainland to Dursey Island |
- |
Cork County Council Appendix D – Post-breeding chough survey report |
|
Isle of Man |
Low genetic diversity |
Reduced viability |
- |
Wenzel et al., 2012 |
A wide range of biotic and abiotic factors could influence chough, notably (abiotic factors) climatic conditions, seasonal weather conditions, nest site availability (buildings, quarries, sea cliffs and cavities within them), and soil type and health (acidity, nitrogen and phosphorus levels), as well as (biotic factors) sward structure and the effects of grazing of different wild and domestic mammals (i.e. land-use), genetics, avermectin or other veterinary pharmaceutical contaminants, soil biodiversity and abundance (i.e. food availability) and its drivers, and interactions with other wild species (including disease, competition and predation). We consider the evidence for all of these below. We do not separate further into biotic and abiotic factors because the lines between them are blurred in some cases, and interactions may be common.
4.1 Land-use and foraging habitat characteristics
The requirement for suitable foraging habitat, close to nesting sites and within the dispersal range of young, will be the single most important factor influencing both the presence and productivity of chough in any given region, as it is for any breeding bird. Chough foraging ecology means that year-round access to insects is critical, chiefly via short-grazed swards, together with the availability of dung invertebrates and, locally, specific habitats such as silage fields, stubbles and the strand line, at different times of year (e.g., Coombs, 1978; Bignal et al., 1989). Of particular importance on Islay are coastal dune systems and cut silage fields, which have been shown to support c.90% and 10% of foraging flocks of chough, respectively (Reid et al., 2009). Within the dune systems, they utilise grazed and largely ungrazed dune grasslands, kelp beds, bare sand, cliff and heath habitats. The sandy areas can be an important source of mining bees, which can provide food for much of the year (Clarke & Clarke, 1995). Newly-cut silage fields provide a rich source of food for fledgling and sub-adult choughs in June to August, when sub-adult mortality can be high. Availability of dung insects is recognised as an important component of chough foraging and diet (Warnes & Stroud, 1989; McKay, 1996; MacGillivray et al., 2018). Hence, land-use providing such resources is a prerequisite for chough presence and persistence in an area, and change in land-use is likely to have a negative impact.
Afforestation is a major current and ongoing form of land-use change. Although its potential effects on the Islay chough population were acknowledged in the 1980s (McKay, 1996), there is no recent evidence to suggest that loss of foraging habitat due to afforestation is driving their population decline throughout the UK and Irish range. However, there are concerns that this may become a significant pressure on Islay in the future. Although there is very little land on Islay that offers more than moderate potential for the establishment of tree crops (Soil Survey of Scotland Staff, 1988), there are signs that carbon off-setting and rewilding could incentivise planting (D. Parish, pers. comm., 2023). Furthermore, there appears to be no evidence suggesting that urbanisation or other forms of non-agricultural land-use are also contributing factors to change on Islay, or elsewhere. However, land-use change that is more subtle may be more significant for chough. In Scotland, increased afforestation around the coast could be a threat to chough foraging opportunities, as a result of both reduced land availability for foraging and increased competition from rooks, especially in foraging areas adjacent to communal roosting or breeding sites.
On Islay, livestock numbers across the island have changed since the 1980s, broadly in parallel with the decline in chough numbers. Fewer farms in key chough areas kept sheep and cattle in 2013, compared with 1989 (Gilbert et al., 2019a), and the average stocking density of farms on Islay is substantially lower than that of other parts in the British chough range with healthier populations (Jonsson et al., 2020). This reflects a long-term trend for reduced stocking densities of both cattle and sheep across Scotland, although numbers may have stabilised recently, to a degree, due to agri-environment and subsidy payment structures, as well as farm economics (Brak et al., 2004; Rural and Environmental Science and Analytical Services Division, Scottish Government, 2021). Further, the relationship between stocking rates and first-year survival rates of chough on Colonsay and Oronsay may be significant. The shift in farm subsidies in 2003 brought a reduction in both sheep and cattle on the islands, which resulted in a widespread loss of the short-cropped turf that is favoured by foraging chough at several cliff-top and dune-system localities. This occurred concurrently with the increase in juvenile chough mortality that supplementary feeding has been shown to mitigate, suggesting a causal link involving food availability (Jardine et al., 2019). While many aspects of biodiversity benefit from reduced farming intensity (Brak et al., 2004), chough foraging requires the short, open swards that high grazing pressure provides and livestock dung further increases the availability of invertebrate food resources (e.g., Gilbert et al., 2019a). Therefore, falling stocking densities, as on Islay, could have had significant impacts on habitat quality for chough. The further reduction in stocking rates is still of concern on Islay and Colonsay, with several local, recent examples of stock removal (D. Parish, pers. comm., 2023). Further losses of stock would likely lead to gross land-use change from land abandonment, with further, but different negative effects on chough. This may be facilitated by the aging profile of farmers on Islay (Brak et al., 2004).
Although the importance of livestock grazing for creating and maintaining short swards and open, bare patches has been established, there has been much less attention paid to other forms of grazing – notably rabbits Oryctolagus cuniculus. McCanch (2000) found that high rabbit populations correlate with large chough brood sizes and foraging success on the Calf of Man (Isle of Man). The strongest correlation was found between the decline in the chough breeding population, and the outbreak of myxomatosis, even when sheep (Loaghtan) grazing numbers were consistently maintained through the study period. Moreover, when the chough population began to recover after 1970, the effects of myxomatosis on the rabbit population were also reducing. The recovery of the Chough population after 1970 corresponds with a change in the effects of myxomatosis outbreaks, probably as a result of the virus being replaced by a less virulent strain, which subsequently became endemic in the local rabbit population. The study suggests that rabbit grazing is as effective as sheep grazing for maintaining the sward characteristics favoured by chough, although they do not contribute to the preferred dung fauna.
4.2 Climate, weather and food availability
In general, British chough, and Scottish chough in particular, are at the northern limit of the species’ range, so climate change might be expected to have presently had, and to continue to have, a positive effect on habitat suitability, allowing northward spread. Moreover, conditions on the edge of the range must always be marginal for a species, all other factors being equal, so we would expect birds there to be the most vulnerable to any stress factors. However, longer-term climate change projections for the UK have indicated wetter winters and drier summers on average, and an increase to the frequency of extreme weather events (Humphrey and Murphy, 2017). Whilst accurate predictions of the severity and location of these weather effects are not available, it is conceivable that changes in weather will be likely to influence chough prey availability and foraging efficiency during periods of heavy and persistent rainfall, especially if they occur at sensitive times of year. Young chough survival rates on Islay are lower in years with higher rainfall totals, when they coincide with the pre-breeding period in late winter and spring, which likely leads to reduced prey availability (Reid et al., 2003; Reid et al., 2008). Specifically, survival of chough fledglings was higher in winters when cranefly (Tipulidae) larvae were more abundant and when previous weather conditions supported increased overall insect prey availability (Reid, 2008). McKay (1996) suggests that the relationship between the climate and the chough’s distribution in the UK probably results from the effects of climate on the growth, mortality and productivity of the invertebrate populations which form their diet. Alternative food sources, such as mown silage fields, low-input arable crops or strand-line wrack, could be critical for buffering local populations against fluctuations in food accessibility in grassland. Although the availability of silage aftermath could be extended by spreading cut dates, these sources are ephemeral or unpredictable, so are only likely to be a supplement, rather than a core resource.
Hayhow et al. (2018) suggests that climatic conditions affecting chough populations in the south (Glamorgan and Pembrokeshire) may have changed to benefit chough in recent years, perhaps contributing to the increases in survival rates and breeding success reported in these areas. Recent mild winters have brought stability to the Pembrokeshire chough population by increasing survival rates of first-year birds. However, extreme weather events in spring and summer can have a severe impact on the population, especially via breeding success. Prolonged strong winds can reduce foraging opportunities throughout the year and heavy rainfall may waterlog nests and kill young. Conversely, prolonged periods of drought can be detrimental for foraging, as the soil may become too dry and firm for chough to feed effectively (Hodges, 1994). In general, such extreme weather events are likely to have a population impact only if they are repeated or prolonged, as numbers may recover quickly if more clement weather allows productivity to improve in subsequent years. Such a change in frequency of extreme events is predicted to occur under climate change; therefore, it is important to consider the possibility that effects of climate will become more apparent and more significant in the future. Note also, that effects could be subtle, such as maritime storm severity and frequency affecting both the accumulation of tideline wrack and its dispersal, perhaps before it can accumulate any invertebrate fauna.
4.3 Use of livestock veterinary pharmaceutical treatments
Following a review of grazing intensities on foraging habitats throughout the chough’s UK range, Jonsson et al., (2020) suggest that higher intensity grazing is preferable, in conjunction with lower rates of avermectin-based treatments for livestock. Macrocyclic lactones, such as avermectins, have long been associated with having lethal and sub-lethal effects on dung-associated invertebrates (Wall and Strong, 1987; McCracken, 1990; McCracken and Foster, 1993). Changes in chough diet on Islay from the 1980s to 2010s, showing a marked reduction in the presence of dung beetle larvae (MacGillivray et al. 2018), are consistent with such an effect following through to an effect on the birds, although this may also reflect the decline in cattle numbers. However, Beynon et al. (2012) found that other parasite-control products (anthelminthics), such as synthetic pyrethroids (SP) (alternatives to avermectins), can also affect invertebrates colonising dung, highlighting the need for testing to be carried out on their environmental effects. In Jonsson et al.’s (2020) review, 63 farms that regularly supported foraging chough were assessed, showing significant differences between chough populations in how farmers graze their land. Farmers on Islay, Colonsay and Oronsay (ICO) were found to administer between four and 13 times more SP treatments to cattle per year than all other regions within their breeding range. Avermectin-based veterinary drugs, for example, have been shown to be lethal to insects inhabiting dung (Lumaret and Errouissi, 2002), and affect many aspects of insect reproduction, including mating behaviour, egg development, oviposition and egg hatching at a sub-lethal level (Strong and Brown, 1987). Webb et al. (2006) suggest that these treatments could affect quantities of larvae present in dung to a degree that could increase the time these dung feeding specialists (e.g., chough, lapwing and redshank) need to spend foraging to obtain sufficient food during key chick development stages. ICO farmers were also found to treat sheep with the highest applications of triclabendazole out of all the other regions in the study (Jonsson et al., 2020). Both SP and triclabendazole products were found to reduce numbers of arthropod larvae found in livestock faeces significantly, during a study into the effects of macrocyclic lactone treatments for livestock on Islay (Gilbert et al., 2019b), although Sands & Wall (2018) found that farms that used SP had similar dung beetle diversity to farms that did not use parasiticides, with their effects being less pronounced than those of Avermectins.
4.4 Genetics
The UK and Irish chough populations are fragmented and isolated, and have been shown to be connected by infrequent and depauperate gene flow, with low genetic diversity reported throughout the north-west European population (Wenzel et al., 2012). Wenzel et al. (2012) reported a genetic diversity gradient from Brittany (highest diversity) to Scotland and Isle of Man (lowest diversity). While there has been some cross-infiltration of chough populations throughout the Irish and UK range in recent decades (e.g., between Isle of Man and North Wales; (Moore, 2006, 2008)), genetic viability remains a concern amongst the smaller populations. Chough in Scotland are closely related, and genetic abnormality is suspected as the cause for blindness in a small proportion of nestlings on Islay and Colonsay (Trask et al., 2016). Most of the Cornish population have descended from the original three birds in 2001 and, consequently, genetic viability continues to remain a concern in the long-term (Mitchell pers. comm., 2021). The extent to which low genetic variability is affecting population dynamics across the UK range is uncertain. There is currently no published, specific, information relating to the genetic viability of the birds used in the Jersey reintroduction programme, but the programme occurs within a context of zoo-based captive breeding, in which management of genetic diversity is a regular focus. Wenzel et al. (2012, 2015) suggest that UK populations are the least diverse in Europe (including island birds in La Palma, Canary Islands, which have been sustained by multiple colonisation events), so may require genetic management intervention to ensure long-term viability. The extent of dispersal among British and Irish birds remains unknown, but could clearly be a critical determinant of genetic population structure. Growing numbers of colour-ringed birds in various populations may offer an opportunity to investigate this further using targeted resighting effort.
4.5 Diseases
A number of diseases are known to affect UK and Irish chough populations. Pseudo-tuberculosis and aspergillosis affecting Scottish chough were first reported by Haycock (1975) and Bullock et al. (1983). Gapeworm (Syngamus trachea) was reported in both wild Scottish (Islay) chough and captive birds in Cornwall (Bignal et al., 1987). The feeding habits of chough make them vulnerable to gapeworm infection as the worm larvae can be transported by invertebrates that form part of the chough diet, including earthworms (Bullock et al., 1983) and leatherjackets (Clapham 1939).The small, isolated population of chough on Islay could be vulnerable to outbreaks of chronic infestations which could have significant impact on productivity and population growth. In addition, gapeworm outbreaks are most common in very wet conditions, which benefit larval survival. It is believed that the particularly wet summer of 1985 in Scotland could have provided optimum conditions for the observed outbreak, while also creating optimum foraging conditions in the soft soil, encouraging gapeworm larvae to the surface (Bignal et al., 1987). Effects of this parasite may therefore be most apparent as relationships with weather conditions, in the absence of surveillance for the parasite itself.
More recently, captive bred chough imported from Paradise Park, Cornwall, to facilitate a captive release programme on Jersey, Channel Islands, are reported to have tested positive for gapeworm. Pre-release birds can be successfully treated; however, two of four birds that had tested positive could not be caught for treatment and subsequently died. Once released, treatment is dependent on whether a bird can be recaptured (Cory et al., 2021).
Although infections with gapeworms are well-recognised in choughs in the UK, other significant internal parasites such as hairworms, gizzard worms and thorny-headed worms have also been found in choughs on Islay since 2004 (Trask et al., 2020). To the best of our knowledge, these parasites have not previously been found in choughs on Islay or elsewhere in the UK.
Note, however, that some incidence of disease is inevitable in wild populations and its existence, per se, does not indicate a clear role in driving population change. Moreover, while disease can cause reductions in numbers, acquired immunity and density-dependent population recovery would often be expected, so critical effects of disease may often only be found when populations are under chronic stress already, such as via interactions with land-use effects. Exceptions include the effect of trichomonosis on greenfinch Chloris chloris, where a novel pathogen spread among very healthy populations at garden feeders and is believed to have driven a rapid population crash (Lawson et al., 2012), but there is no evidence for such extreme disease effects in chough. Nevertheless, although there is no evidence for critical disease impacts, or that the observed incidences of disease organisms are greater than those elsewhere in space or time, contributions of he effects of novel pathogenic internal parasites in choughs on Islay to the decline of this already stressed population cannot be ruled out.
4.6 Disturbance
It is conceivable that frequent or prolonged disturbance at chough nesting sites or foraging areas could have a negative impact on individuals or populations. While some breeding chough inhabit isolated or inaccessible parts of the UK coastline (e.g., parts of Ireland), other populations are located in close proximity to human activity (e.g., Cornwall, Pembrokeshire, Gwynedd and Jersey). As chough expand into new areas and seek new alternative nesting opportunities as a result of population increases or loss of natural nest sites (S. Cummins pers. comm., 2021), human disturbance will become an ever-increasing threat, albeit in the context of an increasing population overall. An eight-year study on human disturbance in a population of chough on a protected, western French island reported a 50% decrease in time spent foraging during the peak tourist season. The study also found that juvenile survival rates were at their lowest during the same period (Keribiriou et al., 2009). Likewise, in west Wales, Owen (1985) found an average 40% reduction in foraging time due to tourist disturbance and that at peak times, around 60% of foraging bouts were disturbed.
A number of potential threats from human disturbance have been documented that are included in a Species Action Plan for the Gwynedd population (see Table 6, for example). Concerns over disturbance have been highlighted for the Pembrokeshire population. In particular, recreational activities around coastal footpaths, cliff-faces and the shoreline may cause disturbance. Although the extent of their effects on survival or breeding success have yet to be measured, these include:
- Disturbance of birds at or in the nest and at resting places used by young and adult birds (climbers, boats/human activity at the base of cliffs below nest sites).
- Disturbance to foraging chough, causing increased vigilance behaviour, resulting in reduced time spent foraging, or foraging in quieter, less productive feeding areas.
- Disruption of general social behaviour, including communal roosting and breeding behaviour. Disruption of social behaviour at roost sites, although effects have not been proven, has been predicted to be especially impactful (e.g., Still 1989).
- Illegal persecution: Shooting and egg stealing.
The significant levels of recreational activity within the Pembrokeshire Coast National Park have been a cause for concern, and safeguarding the chough population from these increasing threats is of paramount importance. The severity of the threat has not been fully assessed and the park authority acknowledge that further investigation is needed (Hodges, 1994). However, the Pembrokeshire population is one of relative stability, so any detrimental effects caused by recreational disturbance are not reflected in overall breeding numbers or productivity. The loss of man-made nest sites through development of abandoned buildings has been raised as an issue on Islay (J.R. Calladine, pers. comm., 2022), and concern over disturbance to nests in active and disused quarries in Wales has led to specific mitigation measures (see Section 5.4: Isle of Man).
4.7 Competition
The degree to which chough compete with other Corvidae species has not been established. Historically, jackdaw (Corvus monedula) was considered to have ousted chough from much of its range (Loder, 1935), however this theory is generally discredited (Bullock et al., 1983). Although their diets overlap to some extent, jackdaws are primarily a surface feeder, whilst chough prefer to probe for food. Where the two species occur together, chough usually forage in the thin, poorer soils (e.g., rough pasture and heathland), whilst jackdaws and rooks (Corvus frugilegus) are more attracted to managed farmland soils of improved pasture or arable (Bullock et al., 1983). However, the increasing proclivity for chough to nest in buildings could put them in greater competition with jackdaws for nest sites, which could cause a problem if nest sites are limiting on local abundance The ability of chough to exploit costal marginal land in favour of typical farmed land would suggest that competition with other corvid species is, to some extent, dependent on how far pasture and arable land extends to the coast (Bullock et al., 1983). McKay (1996) suggests that the earlier breeding by rooks could deplete food resources by the time the later breeding chough fledge young. Although interspecific experimental studies have been carried out between corvid species (Birkhead, 1991), there have been no studies to date that have included red-billed chough. Other species that could conceivably compete with choughs over food include starling and some waders, but this has not been investigated and these species have not increased in abundance. Overall, it is unlikely that competition for resources has had any noteworthy effect on chough in the British Isles.
4.8 Predation
Apart from anecdotal information, there is little evidence identifying the main predators of adult chough, chicks or their eggs. Peregrine falcon (Falco peregrinus) is likely to be the main natural predator of both adult and young birds and has been purported to have caused local extinctions at sites where chough numbers are particularly vulnerable (e.g., two sites in inland Wales in the 1980s (Roberts and Hawkins, 1990)), or population declines in the 1910s on Islay (Baxter and Rintoul, 1953). However, the demise of chough in Cornwall coincided with the decline in peregrine numbers in the same region, which suggests it is unlikely that peregrines are a main cause of chough decline overall. Although it is conceivable that they could have increased the rate of decline, or have caused local extinctions in small and vulnerable populations (Ratcliffe, 1980). Great black-backed gull (Larus marinus) may also be considered as a potential natural predator, especially of juveniles, although there is no evidence to suggest this has contributed to population declines in any regional populations (Bullock et al., 1983). Furthermore, great black-backed gull is also a declining breeding species on the west coast of Scotland (Balmer et al., 2013), so it is unlikely to be a contributing cause for chough decline currently, or in the near future.
It is conceivable that mammalian predators, such as Mustelidae species, brown rat (Rattus norvegicus), or possibly red fox (Vulpes vulpes), occasionally take chicks and eggs, but there is no evidence to support this assumption. These mammals have all been observed to hunt around the cliff edges adjacent to nesting chough in Pembrokeshire, although no quantifiable information has been obtained (Hodges, 1994). For cliff-nesting chough, at least, it is likely that the inaccessibility of most nests acts as the main protection from all types of predators (Bossema et al., 1986). However, this may not be the case where chough nest in buildings and other man-made structures, where nests could be more accessible to a wider predatory threat. Nevertheless, as with disease, predation is a natural, inevitable feature of any and all populations of all species. Natural processes of prey selection behaviour by predators and density-dependent responses of prey populations will tend to prevent long-term declines in prey, unless vulnerability is increased by other, chronic pressures. There have been no formal studies to identify predation as playing a significant role in driving changes in British and Irish chough populations.
4.9 Soil type, quality, health and biodiversity
Soil properties are a critical determinant of vegetation communities and of the invertebrate fauna that lives beneath them. They can determine the availability of insects in soils, irrespective of vegetation and livestock management. Physical and chemical characteristics of the soil (e.g., acidity levels, moisture, temperature, mineral/organic content, contaminants) influence insect abundance and diversity. Further, insect numbers can be significantly affected by the presence of pesticides in both dung and soil (Tucker, 1992). However, no studies could be found that suggest that changes in soil characteristics per se, have significantly influenced chough food availability over time. Moreover, the geology, soil chemistry and hydrology on the Inner Hebrides is particularly diverse (Hudson and Henderson, 1983), which may have helped chough to persist there, through the provision of feeding areas, with soil characteristics that support their prey in a wide range of weather conditions. Future climate scenarios including dry spells in summer may, however, result in prolonged periods of hard, dry soils that are impenetrable to foraging chough.
4.10 Nest site availability
The availability of suitable nesting sites is a factor that may limit the size of a breeding population (Newton, 1994). Along with the importance of suitable foraging habitat, the availability of suitable nest sites for chough (i.e., cliffs, originally) is a major limiting factor for distributions (Newbery, 1998). However, McKay (1996) suggests that the relatively recent proclivity for chough to nest in buildings has in fact provided additional nesting site opportunities. Quarry nesting may have had the same effect, notably in Wales. In addition, Bignal et al. (1987) found no significant difference in average young production per nest between chough nesting in natural nest sites and buildings, so there is no evidence of a change in nesting habitat delivering a form of ecological trap. Overall, a lack of available nest sites is unlikely to explain past chough declines in Scotland, although it could restrict future increases, there and elsewhere. In Ireland, almost a third of all nest locations were recorded from man-made structures during the 2021 Chough census. Cork had the highest concentration with 40% of all known nest sites followed by Kerry with 19%, although it is not known to what extent this is due to greater survey effort of these inland nest sites compared with other regions (Colhoun et al., 2023). Man-made nest sites included cattle sheds, derelict buildings and a barn owl box (Cummins pers. comm., 2021).
5. Review of population establishment and conservation management measures
Management measures for the protection of chough populations or for new population establishment tend to be specific to regional populations, not least because they have often been operationalised by inclusion in agri-environment schemes (AESs), which have been devolved to national administrations within the UK. The approaches that have been used to date are, therefore, described by region/nation below.
5.1 Scotland
Scottish chough are naturally established and were first recorded on Oronsay in 1772 (Pennant, 1774), although Iron Age remains have been found in other parts of the UK, which suggests chough were potentially occupying this region long before the first observational records (Yalden and Albarella, 2009). As a result, they have been subject to conservation measures to preserve an existing population, rather than to establish a new one. The long-term vulnerability of the highly range-restricted Scottish birds has led to the development and operation of a range of AES interventions. Grazing farmland on Islay, Colonsay and Oronsay benefit from three AECS options for chough. These options are delivered through the Agri-Environment and Climate Scheme (AECS) under the Rural Payments and Services, a fourth option is frequently used to manage a mosaic of grazed habitats for chough.
Management approaches in Scotland
Agri-Environment and Climate Scheme (AECS)
The Chough Grazing Management option
The aim of this option is to provide suitable areas for the birds to forage throughout the year by creating short grass swards using cattle, whose dung supports the insects upon which the birds feed. Not treating these cattle with avermectin-based wormers will benefit these insects. This option also allows for an early cut of silage or hay to be taken (before 30th June).
Management requirements - the applicant must either:
- graze the grassland to provide a short sward throughout the year.
or:
- take an early cut of silage or hay, not later than 30 June, and then graze to keep the sward short.
- if cutting, hay or silage must be cut in a wildlife-friendly manner.
For both:
- not treat any livestock with avermectin-based drugs, unless advised by a veterinary surgeon and with prior written approval
- not spray, except for the spot-treatment of injurious weeds (requires prior written notification), or treatment of invasive species (requires prior written approval)
- maintain a diary.
Chough Mown Grassland option
Management requirements
- Must graze the pasture until 14 June.
- Must not treat any livestock with avermectin-based drugs, unless advised by a veterinary surgeon and with prior written approval.
- Must not roll, harrow or graze the field from 15 June until 15 August.
- The area must be mown, but not before 15 August.
- The hay or silage must be cut in a wildlife-friendly manner.
- Do not spray, except for the spot-treatment of injurious weeds (requires prior written notification) or treatment of invasive species (requires prior written approval).
- Must maintain a diary.
Creation of Chough nest shelter option
Management requirements
- Must obtain planning permission, or have confirmation that planning permission is not required, for your proposed nest shelter.
- Must construct the shelter in accordance with published guidance.
- The shelter must be secured or anchored to the ground.
- The nest shelter must include at least one nesting platform inside of approximately one square metre, located in either gable end, or on the centre of the back wall. The location of the platform must be agreed with Scottish Natural Heritage.
- Must not use the nest shelter for storage or livestock housing.
- Must maintain a diary.
Habitat Mosaic Management option
Management requirements
- Must not apply fertiliser, slurry or farmyard manure.
- Must not apply lime, unless you have prior approval.
- Must not allow the land to become poached or vehicle tracked.
- Must not carry out supplementary livestock feeding unless with prior written approval.
- Must not spray, except for the spot-treatment of injurious weeds (requires prior written notification) or treatment of invasive species (requires prior written approval).
- Must maintain a diary.
- Must manage the same location and extent each year for the duration of your contract.
- Must adhere to an approved grazing regime, defining the livestock units and dates.
Supplementary feeding (Islay)
Since 2010, the Scottish Chough Study Group (SCSG) have been running a programme of targeted supplementary feeding on Islay that is designed to target sub-adult birds with the provision of food (mealworms, suet and pinhead oatmeal) at key foraging and roosting localities, to address a pattern of poor first-year survival. A specific protocol for this has not been published.
5.2 Ireland
Chough are on the Amber List of Birds of Conservation Concern in Ireland (Gilbert et al., 2021). The relative population stability and widespread distribution along the northern, western and southern coastlines has negated the need for any direct form of conservation intervention or reintroduction to this region. However, much of the coastline where chough are distributed is served by one or more European conservation designations: Natural Heritage Areas (NHA) and proposed Natural Heritage Areas (pNHA) are the basic designation for wildlife in Ireland, which aims to protect habitats, which support species of concern. SACs are prime wildlife conservation areas, considered to be important on a European, as well as an Irish level. This prime network of wildlife areas is legislated for and designated by the EU Habitats Directive, transposed into Irish law by the European Communities (Birds and Natural Habitats) Regulations, 2011. Coastal habitats, which include machair, a key foraging habitat for Irish chough (presumably when grazed or cultivated, as unmanaged machair would probably quickly become too rank), are also represented under SPAs along the Atlantic and Celtic coasts, which aim to protect rare and vulnerable species under EU law (National Parks and Wildlife Service, 2021). Prescriptive measures to maintain and to enhance habitat areas for chough and increase numbers of breeding chough in targeted areas are delivered through the Department of Agriculture, Food and the Marine (DAFM), via the Green Low-Carbon Agri-Environment Scheme (GLAS) agri-environment scheme.
Management approaches in Ireland
Green Low-Carbon Agri-Environment Scheme (GLAS)
Conservation of farmland birds: chough option
Requirements:
- Produce a suitable sward by developing an appropriate grazing plan to maintain a tightly grazed short sward throughout the year on the areas within the GLAS contract.
- The action must be delivered on full Land Parcel Identification System (LPIS) parcel(s). The LPIS plots selected must be marked on the map submitted with the GLAS application.
- Rolling is not permitted between the 15th March and the 15th July annually.
- Where a parcel is cut for silage/hay, only 1 cut can be taken per year.
- Heather, bracken and scrub, where present, must be controlled where appropriate and only between 1st September and 28th February annually.
- Maximum chemical nitrogen usage is 40 kg N per ha per annum on parcels within the GLAS contract.
The use of pesticides is not permitted. Where noxious and/or invasive weeds are present, they must be controlled preferably by mechanical means; however, spot treatment using pesticides may be required in some circumstances (Government of Ireland, 2021).
5.3 Wales
Chough management in Wales has varied across the subpopulations in different coastal regions. In Gwynedd, north Wales, a three-year conservation programme ran in 2000-2003. This EU funded programme paid for the reintroduction of grazing on coastal slopes, bracken control and the provision of overwinter cereal stubbles - primarily for the benefit of chough (Pontcymru, 2021). It is important to note that chough nesting in this region are largely dependent on historical mining sites and (often disused) quarries, in addition to coastal management incentives. Coastal grazing has also been implemented on grassland around the Llyn peninsular coastline through the Llyn Landscape Sustainable Management Scheme and National Trust Payment for Outcomes Scheme. On the Gower peninsula (south Wales), key habitats are on common land. Commons graziers have worked closely with the National Trust in order to manage the cliffs to specifically benefit chough. Alongside this, cattle are treated with non-avermectin-based drugs, and cliff-top bracken and scrub are controlled to encourage livestock grazing. This in turn promotes dung insects and short turf for chough and their young (National Coast Watch, 2021). From 2012 to 2020, prescriptive land management measures for chough (for all of Wales) were delivered through the Welsh Government’s whole-farm sustainable land management scheme, Glastir. Replacement measures are expected under the new Sustainable Farming Scheme in 2022, but have yet to be published; currently, agri-environment management in Wales is in hiatus following the UK’s departure from the European Union.
Management approaches in Wales
Glastir (2012-2016)
Grassland management for chough (feeding) option
Requirements:
- The rules for habitat under the Glastir Whole Farm Code apply to all the land within this option.
- Maintain as grassland by grazing.
- At least 80% of the sward must be between 3cm and 5cm high throughout the year.
Landowners must not:
- Clear out existing ditches.
- Apply any insecticides, fungicides or molluscicides.
- Apply lime or any other substance to alter the soil acidity.
- Cut or top vegetation except to control injurious weed species.
- Burn vegetation or other materials.
- Roll or chain–harrow.
- Supplementary feed livestock.
- Plant trees.
- Carry out any earth moving activities.
- Use for vehicle activities or as a track.
- Construct tracks, roads, yards, hardstandings or any new structures.
- Store materials or machinery (Welsh Government, 2020).
Species Action Plan for the Gwynedd Chough population
Habitat management and species protection objectives
Requirements:
- Limit disturbance caused by recreational access near nest sites or communal roosts by liaison with British Mountaineering Council.
- Safeguard all potential nest sites and communal roosts from inappropriate development, especially mine capping, grilling or reclamation.
- Seek to limit access to any nest sites in an appropriate manner where egg collecting is a threat and where public access cause continual failure of the breeding pair.
- Maintain pit props or other man-made structure where they are a key feature of the nest site. Ensure maintenance of artificially installed nest sites – nest boxes and other artificially-made and strengthened sites.
- Keep mine entrances and quarry sites free from scrub or trees.
- Ensure that all nest sites in working quarries are identified and that during the breeding season nests are not destroyed, nor adults seriously disturbed by quarrying activities. (Lamacraft, 2004).
Chough in Pembrokeshire
Current conservation measures (undertaken between five partner organisations)
Requirements:
- Input into the Environmentally Sensitive Areas (ESA) Scheme: Advisory and monitoring role.
- Management of reserves, with specific prescriptions designed for chough.
- Coastal Management Initiatives and management agreements with farmers.
- Management of coastal properties.
- Management of the nature conservation/recreation interface: safeguarding of nest sites, (e.g. agreed seasonal ‘cliff 22 Chough Conservation Strategy for Pembrokeshire’ climbing restrictions).
- Maintenance of open sea-cliff communities (e.g. Armeria/Festuca grassland and therophytic communities on eroding slopes and cliff edges).
- Structural variety: mosaics of vegetation, rock and bare earth, and areas enriched by dung.
- Low intensity mixed farming regimes: grazing of cliff lands supplemented by ‘cool’ burning (practice of the selective, closely controlled burning of scrub or bracken to create open areas of short vegetation), winter cereal stubbles and out-wintering of cattle on coastal fields.
- Unploughed field headlands, untreated with pesticides.
- Avoidance of the use of avermectin and other anti-parasitic chemicals on cattle grazed in coastal fields and on cliff headlands (Hodges, 1994).
Specific land management prescriptions applied are:
- Restoration and maintenance of traditional (mixed) grazing on cliff-tops, coastal slopes, headlands and sand dunes.
- Provision of winter cereal stubbles (either in fields or in strip cultivations) in adjoining coastal fields.
- Management of scrub and bracken on coastal slopes and sand dunes in targeted areas (by cutting or grazing) to favour short turf or heath.
- Management of heath for chough, including cutting and/or controlled ’cool’ burning as well as grazing.
- Creation of mosaics, e.g., bare earth/vegetation/rock interfaces, edge habitats.
- Maintenance of earth/stone banks in such a way as to allow chough access to feed at bases and on the tops, e.g., grazing, cutting where necessary.
- Encouragement of de-intensification of the farming regime in selected coastal fields, to improve their potential as feeding habitats for chough.
- Encouragement of winter grazing in coastal fields as well as on cliff lands, especially by cattle.
- Management of the interface between people in important areas for chough, e.g., by using seasonal footpath diversions in sensitive areas, widening the coastal corridor (by removing the seaward fence) to create more room for stock, people and chough in selected areas, and the operation of agreed seasonal cliff climbing restrictions in the vicinity of nest site.
- Encouragement for the reduction of the use of avermectin and related chemicals as a prophylactic in cattle in target areas, through appropriate agricultural support mechanisms.
- Consideration to be given to the provision of artificial nest sites in physically and ecologically appropriate locations - where there is a demonstrable need for these.
- Provision of advice on all aspects of land management for chough to landowners, farmers and managers, through the various schemes and initiatives listed above (Hodges, 1994).
5.4 Isle of Man
The Isle of Man chough population is descended from naturally occurring birds and has not been reinforced with reintroductions. The relative stability of the population has not necessitated conservation intervention measures, such as supplementary feeding, or any form of captive releases. Agri-environment measures are delivered through the Agri-Environment Initiatives Scheme (AEIS), which falls within the Agricultural and Fisheries Scheme (AFGS). There are currently no specific measures or options prescribed for the conservation management of chough, other than the AES nest box option (5/07) for chough/barn owl. However, chough feature in the criteria for the Isle of Man government guidelines for selecting Areas of Special Scientific Interest (ASSI), and are listed as a species which benefit from several protected grassland communities (Isle of Man Government, 2008).
Management approaches in the Isle of Man
Nest box option conditions:
- Each location must be agreed with the Agri-Environment Delivery Partner prior to installation.
- Each box must be kept in its original location for a minimum of 5 years.
- Nest boxes should be in an accessible barn.
Nest boxes should be at least 3m high with a shelf to prevent young birds falling.
5.5 England
Chough in England (Cornwall) are a newly establishing population. The first pair to arrive in Cornwall in 2001 is believed to have originated from the Irish population (CBWPS, 2021). Prior to this, a captive breeding programme had been operating since 1976 and a first trial release was conducted in 2003 to add to the natural colonisation, following many years of captive breeding and viability studies at Paradise Park wildlife sanctuary. The reintroduction programme, ‘Operation Chough’, has developed a strategy to keep the Cornwall population viable by monitoring nesting areas, promoting awareness of the cultural and environmental benefits of the species to Cornwall and by maintaining a captive bred population to further enlarge the genetic pool of the pioneer communities if required. Initially, six birds, (originating from Welsh stock) were released, with two pairs surviving until late November, at Trevean near Zennor, although one pair was illegally shot dead by a local resident (Hales, 2021). However, the present Cornish population solely originates from the three individuals that naturally recolonised in 2001, confirmed through DNA analysis, so the reintroduction of captive-bred birds did not succeed in terms of contributing directly to the population. Two of the three individuals subsequently bred the following year and the third bird (female) subsequently bred with the male offspring from the first pair. In 2006, this second pair successfully bred and raised two chicks (H. Mitchell, pers. comm., 2022). It is not clear what has limited the success and integration of introduced birds, but it could involve some combination of assortative mating and lack of local adaptation or genetic fitness among introduced birds.
Long-term population persistence seems likely to require additional inputs of genetic diversity. Operation Chough will continue to assist the fledgling local population and monitor its progress. It will make captive-bred birds available for release in Cornwall to secure the future of the current population, if necessary for demographic or genetic reasons, and will also pursue its further objectives:
- Increase the captive population by extending partnerships with bird collections, and establishing captive breeding groups in several locations.
- Investigate previous historic sites outside of Cornwall to identify where chough could be re-established.
- Make birds available for release elsewhere where this will support further colonisation.
- Promote work on chough genetics (Hales, 2021).
A small breeding colony is now naturally established with the support of various conservation organisations and initiatives. Soon after the first pair arrived in Cornwall, a suitable network of priority habitats were identified along the Cornish coastline based on former breeding localities, areas with existing quality foraging habitat, and areas with potential for future habitat restoration. A dedicated project team (The Cornwall Chough Project) was implemented to safeguard and monitor the population (Carter et al., 2003). The project aims to ensure the future for chough by working with landowners to restore habitats with appropriate management along the coast. This includes the use of a selected mix of native cattle, ponies and sheep to create a mosaic of open, short sward to enable foraging for invertebrates, as well as other targeted agri-environment measures (see Management approaches in Scotland). A dedicated team of volunteers have been monitoring nests since the birds first arrived (RSPB, 2021). The National Trust ‘Grazing Initiative’ has re-established grazing on coastal grassland using ponies, sheep and cattle to create a mosaic of vegetation structures for the benefit of chough and other taxa, in conjunction with the agri-environment scheme measures (Rylands et al., 2012). There are currently no specific options available through government-led agri-environment schemes that are prescribed for chough in England, no doubt as a result of their restricted English breeding range, although particular management measures would be supportable via management for special, local features (in this case, chough), as agreed between agreement-holders and Natural England representatives (see Management approaches in England). RSPB (Rylands et al., 2012) produced a report to assess the management needs for chough and other priority species along the Cornish coast. The highlighted measures were adapted from the chough conservation research conducted on Islay (McKay, 1996). Following the UK leaving the European Union, a new approach to agri-environment support is in development, through the Environmental Land Management Scheme (ELMS). The tier of ELMS called Countryside Stewardship Plus (CS+) is the most likely to be relevant to chough, as it will include provision for farm holdings to undertake specific management measures that are appropriate for the location. It will add around 30 new options to those in the existing CS scheme and will be developed further through 2023 and 2024, but no options to date specifically refer to chough, so relevant management is likely to involve local variants of generic grazing options (DEFRA, 2023).
Finally, note that a plan to reintroduce chough to Kent is well under way, and the first planned release is set for summer 2023 (Kent Wildlife Trust, 2021). To date, this reintroduction has produced no concrete examples of management to contribute to the present review, but it plans to build on the expertise developed in Cornwall and applied successfully to the reintroduction in Jersey (see section 5.7: Jersey; Kent Wildlife Trust, 2021).
Management approaches in England
Higher Level Stewardship – Environmental Stewardship Scheme 2005-2016
Management of grassland for target features
These options were used to manage grassland for target features including chough. The Farm Environment Plan (pre-agreement assessment) included identification of features like local chough that would benefit from management under grassland options:
HK15 Maintenance of grassland for target features £130 per ha
HK16 Restoration of grassland for target features £130 per ha
Aim: to maintain or to restore semi-improved or rough grassland, which is known to provide good conditions for target species, including moderately species-rich, semi-improved and enclosed unimproved grassland.
HK17 Creation of grassland for target features £210 per ha
Aim: to create semi-improved or rough grassland on former arable, set-aside or temporary grassland.
Managed as HK15, following creation by establishing a grassy sward through natural regeneration or by sowing a seed mixture recommended by your Natural England adviser.
Prescription:
- Not specified and intended to be flexible, for agreement with local advisers. In practice, guidance would probably follow evidence from elsewhere, notably research from Islay.
The National Trust ‘Grazing Initiative’ (Cornwall)
Re-establishment of coastal grazing of grassland and heath using hardy, native breeds of pony (including Dartmoor), sheep and cattle (including Highland) for the benefit of chough and other taxa. The area and timing of grazing is not fixed, so will be different each year. Ponies will be removed at the end of February to protect wild flowers in the spring.
Prescription:
- Not specified and intended to be flexible due to the requirement to safeguard other plant and animal species specific to Cornish coastal habitats.
RSPB Summary of suggested chough land management requirements (adapted from McKay, 1996)
Favourable to chough:
- Short vegetation.
- Diverse pastoral habitats: a mixture of permanent pasture, improved pasture, rough grazing dry grassland (e.g. dry heath/acid grassland), arable farmland, sand dunes and beach strand lines.
- Low-input spring-sown cereal crops producing winter stubbles.
- Permanent established/regular grazing.
- High grazing pressure (without high fertiliser input) to produce a short sward and exposed soil.
- Hay/silage ‘aftermath’ growth (beneficial for only 1-2 weeks after cutting unless grazed).
- Mixed stocking (greater variety of herbivore dung and grazing techniques).
- Out-wintering of stock (especially cattle).
- Cornish hedges/Earth bank field boundaries - especially unfenced.
- Farm yard manure production, manure heaps, muck spreading.
- Use of non-avermectin based de-worming drugs.
- Rabbit grazing.
- Intact pastures.
5.6 Northern Ireland
All birds in Northern Ireland are afforded the same IUCN criteria as Ireland (Republic), chough, therefore, share the same conservation status and are on the Amber List of Birds of Conservation Concern (Gilbert et al., 2021). Prescriptive land management measures have been introduced along the Antrim coast as part of the Antrim Coast and Glens Environmentally Sensitive Area Scheme. Landowners within the scheme can sign up to a chough management option within the current agri-environment scheme (the Environmental Farming Scheme [EFS]). In addition, RSPB, National Trust and local farmers have all contributed to the creation of more suitable conditions for chough in north Antrim (Allen and Mellon, 2021).
Chough management is now primarily delivered through the ‘coastal grazing option’ in the Environmental Farming Scheme (EFS), following the similar option in the predecessor Countryside Management Scheme (CMS), the aim of which is to maintain and increase coastal grassland for a range of coastal birds. This option is implemented on a field-by-field basis, depending on the suitability of the field.
Management Approaches in Northern Ireland
Countryside Management Scheme (CMS) 2007-2013
Species rich dry and species rich calcareous grassland
Applies to dry and calcareous grassland with five or more wild flowers, native grass or sedge species, indicative of dry/calcareous conditions, found at least six out of ten random 1m2 quadrats in a field. Indicator species include bird’s foot trefoil, thyme and lady’s bedstraw, with less than 25% ryegrass, timothy and white clover in the sward.
Aim: maintain and enhance the conservation value of species rich grassland through appropriate agricultural practices such as positive grazing management and restrictions on fertiliser and pesticide use. Biodiversity objectives include chough, among other priority species.
Prescription:
- Land must be kept in ‘Good Agricultural and Environmental Condition’ (GAEC; EU standard).
- All year grazing option: Year-round grazing at a stocking density of 0.5 LU/ha or
- Restricted grazing option: No grazing is permitted between 1 May and 31 July. Grazing is permitted between 1 August and 30 April at a stocking density of 0.75 LU/ha.
- Land must be maintained by grazing. Excess grass may be saved for hay or silage but must not be cut until after 15 July.
- Cultivation, chain harrowing, fertilisation, reclamation, mineral extraction, dumping, infilling, new drainage or construction of new lanes are not permitted.
- Application of slurry, chemical fertiliser, lime, herbicides, pesticides, insecticides, sheep dip, fungicides, basic slag, sewage sludge, poultry litter or any other material is not permitted.
- Where farmyard manure has traditionally been applied, annual applications must not exceed15 kg N/ha or 2.5 t/ha.
- Supplementary feeding sites, temporary silage clamps and storage areas for big bale silage or hay are not permitted.
- The spread of scrub/trees must be controlled. Routine positive management such as scrub control and noxious weed control (by cutting between 15 July and 15 March, or by spot-spraying herbicides) are included
- Trees must not be planted on species rich grassland.
- No rolling is permitted in April, May and June.
- No poaching is permitted
- Existing drainage systems can be maintained, but not widened, deepened or extended.
Coastal grazing option
Applies to coastal grassland, with an individual plan for each field entered into this option. The most suitable management for each field was decided at the outset of the agreement.
Aim: maintain and increase coastal grassland for a range of coastal birds. The primary biodiversity objective was to contribute to the Biodiversity Action Plan targets for the chough.
Prescription:
- Land must be kept in GAEC.
- Grassland must be maintained by grazing or cut for hay or silage.
- Fields entered as coastal grazing option must be kept in this option for the duration of the agreement.
- This option applies to improved, semi-improved and semi-natural grassland.
- Annual application of Nitrogen (N) must not exceed 100 kg N/ha for semi-improved grassland and 25 kg N/ha for semi-natural grassland.
- Grassland fields must be grazed to create a sward height of less than 2-3 cm for a specified period. This should be achieved through mixed grazing, grazing some fields all year round, topping of grass tussocks and staggering silage cutting dates.
- Spreading scrub/trees, particularly gorse (whin), must be controlled.
- Scrub must be removed where it has encroached on to coastal grazing option land.
- Rushes must be controlled by cutting only between 15 July and 15 March. Herbicide control is not permitted.
- Application of pesticides, herbicides, sheep dip or any other material is not permitted.
- Noxious weeds may be controlled with herbicides, applied using a weed wiper or spot sprayer.
Other options
Note that the CMS also included a range of options for which chough was named as a target, but for which the primary aims were other species, notably including all options that were named as contributing to the arable field margins Habitat Action Plan. These are not detailed here as they are of limited relevance for chough management. The options concerned were lapwing fallow plots, undersown cereals, retention of winter stubble, conservation cereal and wild bird cover. Detailed prescriptions are in Northern Ireland Rural Development Programme (2007).
Environmental Farming Scheme (EFS) 2020 onwards
Chough Grazing Option
Requirements:
- Land must be kept in GAEC.
- Grassland must be maintained by grazing or cut for hay or silage.
- Fields entered as coastal grazing option must be kept in this option for the duration of the agreement.
- This option applies to improved, semi-improved and semi-natural Grassland.
- Annual application of Nitrogen (N) must not exceed 25kg N/ha for semi-natural grassland.
- Grassland fields must be grazed to create sword height of less than 2-3cm for a specified period. This should be achieved through mixed grazing, grazing some fields all year round, topping of grass tussocks and by staggering silage cutting dates.
- Spreading scrub/trees, particularly gorse, must be controlled.
- Scrub must be removed where it has encroached on the coastal grazing option land.
- Rushes must be controlled by cutting only between 15 July and 15 March. Herbicide control is not permitted.
- Application of pesticides, herbicides, sheep-dip or any other material is not permitted.
5.7 Jersey
In 2010, captive-bred birds from Paradise Park, Cornwall, originally bred from Welsh stock, were sent to the Durrell Wildlife Conservation Trust to begin a reintroduction programme with the intention of establishing a free-ranging population on Jersey. The trial release started in 2013 using six closely monitored birds. By 2021, there were 10 successfully breeding pairs. Nine of the pairs comprised parent birds reared at either Jersey Zoo or Paradise Park, and one pair were both wild-hatched. Four of the captive bred parent birds were hand-reared as chicks. As of 2021, the total Jersey flock consists of 12 males and 18 females (Corry, 2021).
The captive breeding programme is the result of development of successful methods over 30 years, including important lessons learned regarding effective practices (Burgess et al., 2012; Operation Chough, 2021). There were important determinants of success in the breeding programme, such as provision of live food, especially ant eggs, small mealworms and crickets, in the first days after hatching, siting aviaries away from human disturbance, allowing chough to choose partners themselves in winter groups, and close monitoring via nest cameras to identify health problems and to facilitate early treatment. Artificial egg incubation and hand rearing can be worthwhile, but close monitoring can prevent the need for this. Importantly, over 30 years, eggs were laid in 77 nests, but only 27 produced young and only 48 fledglings were produced in total (Burgess et al., 2012), illustrating that providing chough for introduction into the wild is not straightforward.
Post-release, Jersey chough have been supported with some supplementary food, presumably involving the same, balanced diet on which they are kept in captivity, but details on the protocols that have been used have not been published (Birds on the Edge, 2014). Habitat management has also been critical: much cliff top farmland in Jersey has been abandoned and has become dominated by bracken, so active management to remove it, following specific grazing management, aims to restore grassland for chough foraging, with chough acting as a flagship species for the system (see Management Approaches in Jersey; Birds on the Edge, 2014a, 2014b, 2014c).
Management Approaches in Jersey (by the National Trust for Jersey)
Post-release habitat management
Bracken removal
Aims: to reduce extent of bracken coverage on coastal areas in order to restore native coastal grassland and heathland vegetation and to restore coastland bird and other animal populations.
Bracken is being removed using methods that are most appropriate for each individual site:
Cutting – during early summer, before and up to the point of maximum frond expansion. The aim is to ensure a maximum withdrawal of carbohydrates and nutrients from the rhizome reserves. Fronds should be cut in late July/early August but cutting may be carried out one, two or three times annually. This will not eliminate bracken but will reduce its vigour and prevent further encroachment.
Bruising – can be more effective than cutting because the plant continues to send nutrients into the bruised/damaged part of the plant rather than sending up new shoots as happens following cutting. Bruising should be carried out in June/July when fronds are emerging and when the plant is withdrawing energy from the rhizome. Follow-up treatment in July and August will provide maximum control. Bruising should take place in successive seasons to exhaust the plants.
Spraying – herbicide action is unlikely to have a significant effect on the amount of rhizome carbohydrate reserves, so herbicides which attack frond buds on the rhizome are most successful. Commercial herbicides using Asulam [methyl 4-aminobenzenesulphonyl carbamate], which is specific to fern species such as bracken and is less likely to affect other vegetation or wildlife, are the most widely used herbicides for bracken control. Asulam frequently produces a very good reduction in fronds in the year after spraying, but there is often rapid frond recovery unless other treatments are applied in following years, so multiple applications of Asulam are more effective than single applications. Follow up spraying, once cover has been reduced, may be spot sprayed with a knapsack and lance. Alternatively, weed wiping may be appropriate. Spraying may also be more appropriate where there are ground nesting birds or reptile populations present which could be damaged by mechanical control methods.
Livestock grazing – livestock will consume small amounts of bracken but not in sufficient quantity to reduce cover significantly. Importantly, bracken is toxic to livestock when consumed in quantity, and so livestock grazing should not be used as a method for bracken control due to welfare concerns. However, livestock (cattle and ponies) can be a useful management tool through trampling. Sheep may offer some control of bracken once initial control has been undertaken; where there is a grass sward beneath the bracken, management to remove the tall bracken may allow sheep to graze the sward, exerting trampling pressure on the regenerating bracken and help to maintain the grass.
Litter removal – dense stands of bracken may have a thick layer of litter that will smother regenerating vegetation, whilst containing rhizomes and protecting them from frost and drought. Ideally, this layer should be removed to speed up restoration of the ground vegetation. In small areas this can be achieved by raking by hand, or with a forage harvester (on flat sites). Alternatively, implements such as discs or rotovators can be set at a depth to break up the litter but leave the mineral soil profile undisturbed.
Grazing
Aims: to re-establish grazing flocks of Manx loaghtan sheep to achieve effective and sustainable land management, which in turn will enhance the habitats on Jersey’s cliff tops, to restore target biodiversity.
In 2008, the National Trust for Jersey introduced sheep, supported by a shepherd, to build the sheep flock to a sustainable size over a five-year period. Livestock grazing should control more competitive plant species, and prevent scrub encroachment and succession, with extensive grazing supporting a vegetation mosaic. Animal dung and areas of bare ground produced in hoof marks also provide resources for some specialist invertebrates and birds (including chough). Mechanical and chemical management has been used to facilitate the restoration process. In the long term, the density and timing of livestock grazing needs to be tailored to the needs of an individual site; even within livestock species, individual breeds can graze differently.
6. Evidence for the effects of management interventions on chough abundance and population dynamics
Review of the range of types of intervention for chough, that are described in Section 5: Review of population establishment and conservation management measures, indicate that the practices that have been used to date can be collated into the following eleven key, general categories:
- Habitat designation
- Creation of short swards and open ground for foraging
- Enhancement of dung availability and quality
- Supplementary feeding
- Release of captive-bred birds
- Provision of nest boxes or shelters to enhance breeding
- Low-input spring-sown cereal crops producing winter stubbles
- Maintenance of earth/stone banks for chough foraging
- Avoidance of disturbance and development around nests and roost sites
- Unploughed field headlands, untreated with pesticides
- Provision of advice to land managers
- Anthelmintic drugs
The evidence relating to each of these is considered below. In addition, the ecological knowledge that has been gained about chough on Islay, in particular, may suggest further approaches that have not been applied in formal schemes or programmes. Such possibilities are considered in Section 6.12: Anthelmintic drugs. A general limitation with the evidence that is available is that there have been few, if any, tests of the effectiveness of the individual interventions, as opposed to programmes that combine multiple elements. This makes the unique importance of specific measures, as they are applied in practice, impossible to evaluate. The evidence for likely effectiveness therefore relies largely on observations of associations with habitat or agricultural practices. Hence the real effects of changes in practices or habitat structures can only be inferred from associations with the land-uses involved (Section 2.4: Review of evidence for the effects of management interventions on chough abundance and population dynamics), so the evidence available is no more than indirect. This is an important caveat, because effectiveness of management actions is very often dependent upon the details of context and application, which also underlines the importance of ongoing monitoring: effects in principle do not necessarily translate into effects in practice (e.g. Baker et al., 2012, Siriwardena et al., 2008, Chamberlain et al., 2009). Note also that much of the evidence for the possible effects of management measures therefore relates to fundamental biotic and abiotic influences and so is reviewed in Section 4: Review of the evidence for the influences of biotic and abiotic factors (and interactions between them) on chough population dynamics.
6.1 Habitat designation
As a scarce, range-restricted species in Britain and Ireland, chough has attracted conservation measures via habitat designation under a range of schemes, such as Special Protection Areas (SPAs), Environmentally Sensitive Areas (ESAs), Natural Heritage Areas (NHAs, Ireland) and Special Areas for Conservation (SACs). For example, chough is a cited species and a feature of European importance for three SPAs in Pembrokeshire (Ramsey and St David’s Peninsula Coast SPA, Skomer, Skokholm and the Seas off Pembrokeshire SPA and Castlemartin Coast SPA). Such habitat protection measures may be necessary and common, but demonstrating their effects is difficult, because benefits depend on threats actually occurring. Evidence for the effects also requires counterfactual monitoring, i.e., finding matched protected and unprotected areas for comparison, which is rarely possible because all suitable habitat areas are protected. Positive effects may only be seen via population persistence within the designated areas, which may be welcome, but, alone, does not indicate that local populations are healthy and exporting offspring elsewhere, for example. Specific management activities may therefore be needed to improve habitats; indeed, designation may require active management to maintain site/area condition, because threats to maintenance of, for example, extensive farming, arise because practices are uneconomical, so would not persist without support. Designation may therefore best be viewed as an important step, but not necessarily effective unless combined with other measures as are considered below.
6.2 Creation of short swards and open ground for foraging
Areas of bare ground and short vegetation have been identified as a key factor for chough foraging success (Bignal et al., 1989; Johnstone et al., 2011). The majority of chough management approaches that have been applied to date involve enhancing habitats in which chough can forage successfully, reflecting the identification of this factor as key for chough survival and productivity throughout the UK and Irish range. The requirement to access bare ground and short grazed coastal grassland and heath has been well-documented (e.g., Warnes and Stroud, 1989), and the need to sustain this niche foraging habitat is undeniably important for accessing invertebrates on or just below the soil surface.
Mosaic habitat consisting of short vegetation and small patches of bare ground has also been found to be particularly favoured by chough (Roberts, 1983; Kerbiriou and Le Viol, 1999; Rylands et al., 2012). In one study, grazed vegetation up to 2cm was found to be the most suitable habitat for accessing prey, followed by soft, bare ground (Whitehead et al., 2005). On Islay, chough prefer to forage in short grass and exposed soil on dune pasture, where there is greater accessibility to soil invertebrates. Tipulid larvae taken from dune pasture soil, make up a significant portion of the chough diet in early summer in this habitat, after which they pupate and emerge as adults; dung-feeding invertebrates are important all year round (MacGillivray et al., 2018).
Management regimes that produce these habitats have therefore been widely adopted with the aim of benefiting chough. Providing there remains an availability of sufficient areas of short vegetation and bare ground, conditions provided by mixed grazing regimes have been shown to work favourably for chough overall (Rylands et al., 2012). Across the range of prescriptions that were identified in section 5.0: Review of population establishment and conservation management measures, the following specifics have been used, although some are contradictory to some extent:
- use a selected mix of native cattle, ponies and sheep to create a mosaic of open, short sward (but 3-5 cm or <2-3cm) to enable foraging for invertebrates;
- use high grazing pressure;
- limit fertiliser, but <40kg N/ha, <100kg/ha, <25kg N/ha or no fertiliser at all;
- use no supplementary feeding of livestock;
- no harrowing, or only limited harrowing;
- use low-intensity silage/hay management: only one cut per year;
- remove/control heather, gorse, bracken, rush and scrub, using multiple methods, including selective burning, cutting, bruising, litter removal, grazing;
- control weeds mechanically or by spot-spraying, or use no pesticide inputs.
There have been no formal tests of the efficacy of these approaches for chough. Note also that the species-rich grassland option in the CMS in Northern Ireland (Box 5.6), which had chough as a stated target, has conflicting management requirements to those listed above, notably with respect to grazing pressure, so is not compatible or interchangeable with ideal sward management for chough as most schemes define it. Management in a given location may therefore be a choice between the two different conservation targets.
There is one, rare example of a direct test of the effects of agri-environment management, considering grassland options in Wales, as part of a multi-target assessment of the historical Tir Gofal scheme (MacDonald et al., 2019). Chough productivity data were acquired from the Cross and Stratford Welsh Chough Project. Territories from this dataset were considered if they had been occupied for at least one year between 2000 and 2009, and response variables of nest site occupancy and estimated fledgling number were modelled against the proportion of land within a 300 m buffer of the nest site that was managed under Tir Gofal (which included chough management similar to that under Glastir; see Box 5.3: Management approaches in Wales). Chough were also surveyed in the non-breeding season, across 67 to 239 fields in each of six large sites in one winter, comparing chough densities in Tir Gofal and non-Tir Gofal fields. However, this study found no statistically significant effect of the agri-environment management on either of the two breeding chough variables, and indeed found a marginally negative association between chough-specific Tir Gofal management and winter chough counts. Too much should not be inferred from this result, because a range of confounding factors may have affected the responses, such as the inclusion of a wide range of alternative foraging habitats, which was not considered in the analyses, or that the population considered is healthy and therefore perhaps not in critical need of management support. Habitat selection in the breeding season or, critically, post-breeding, when young chough on Islay are most vulnerable, was also not considered. However, it may argue against a strong benefit of Tir Gofal grassland management for chough.
A second specific evaluation of grassland land-use effects on chough involved a before-and-after study at South Stack RSPB Reserve, Anglesey, Wales (Ausden and Bateson, 2005). This showed that, following the introduction of year-round cattle grazing (<1 ha-1 in winter, 2.5 ha-1 in summer) from spring 2002, chough use of an area of 26 ha of semi-improved grassland increased. This change was associated with a large reduction in sward height, although this was only a very small-scale study.
6.3 Dung availability and quality
Dung from large herbivores can provide a valuable source of food for a number of bird species, especially chough, and offers additional feeding opportunities when ground conditions are less favourable for foraging. If available, chough will forage in dung all year round, although McKay (1996) found summer and autumn to be the most important as a food resource for first-year birds. On Islay, the availability of all-year-round livestock dung, as the result of grazing overwintering cattle, has been found to be particularly beneficial for chough (McCracken, 1990). Dung invertebrates also form the majority of prey biomass in dune pasture on Islay (MacGillivray et al., 2018). Firm, fibrous cow dung is favoured more than dung with more of a liquid consistency. Cow breeds such as Galloway and Dexter are reputed to be more likely to produce these types of pats compared to Friesians, for example (Rylands et al., 2012).
Livestock grazing to deliver dung invertebrate resources to promote foraging should therefore be a clear, straightforward management measure to benefit chough, subject to the sward being maintained in a suitable state (Section 6.2: Creation of short swards and open ground for foraging). Limits will therefore apply in terms of the densities of grazers that can be sustained on unfertilised pasture. Specific variations to grazing management in general that have been proposed in management schemes (section 5.0: Review of population establishment and conservation management measures) include mixed stocking and out-wintering of cattle. Further, manure spreading could also deliver benefits, although practical issues would include sourcing manure in regions where intensive grazing is not practised nearby and the unintended consequences of fertilising extensive pasture. It will also be critical to avoid the use of anti-parasitic treatments like avermectin on the livestock involved: this aspect of management of the dung resource is common in management prescriptions, but has not been evaluated specifically.
However, there have not been any formal assessments of dung-focused interventions for chough, investigating for example the relative importance of dung and sward structure, although the distinction may be moot as grazing management will inevitably influence dung levels and quality, so it is probably best considered and measured as a component of habitat management.
6.4 Supplementary feeding
Supplementary feeding of chough has been employed specifically to benefit the survival of young birds. The Scottish Chough Study Group (SCSG) have conducted targeted supplementary feeding on Islay since 2010 to provide sub-adult birds with mealworms, pinhead oats and suet at key foraging and roosting localities, in order to address an acute problem of critically low first-year survival that was identified in chicks hatched in 2007 and 2008. Following a successful trial at a single locality (Ardnave), with an ‘unfed’ group located at Kilchoman acting as a control population, this supplementary food was also introduced at Kilchoman and additional sites (Bignal and Bignal, 2011) and has continued since, with encouraging counts of young birds being recorded using food and recruiting to the breeding population (Bignal, 2021). Adult survival also increased significantly with feeding (Fenn et al. 2020; Trask et al. 2020).
It appears that supplementary feeding has proved successful in this instance, addressing a major food resource crisis, but there is no evidence to date that such a measure would be useful in other populations, i.e., that food effects on first-year (or adult) survival are a critical population limit in other parts of the range. Feeding also only offers a short-term solution to the problem of food availability for first-year birds, as it requires ongoing effort and intervention. A self-sustaining solution that is integrated with local land management would be preferable, as is acknowledged by Trask et al. (2020). Effective grassland habitat management should negate the need for such forms of intervention and clearly the management that was in place before the population declined supported chough foraging adequately, so this is possible. Low food availability, in conjunction with high parasite loads and low genetic diversity, should be tackled simultaneously to address the problem of first-year mortality (Trask et al., 2020).
6.5 Release of captive bred birds
In the absence of natural colonisation (cf. the Canary Islands context, Wenzel et al., 2015), increases in genetic diversity can only be achieved by translocation of some kind. In principle, adult wild birds could be translocated directly from other populations, or eggs could be moved or swapped between nests in different regions, but these approaches do not appear to have been trialled. Moving eggs or adult birds between threatened local populations would be a high-risk strategy, as rejection by parents or failure to adapt could have significant conservation consequences. Success also seems less likely if donor and recipient populations are further apart or more dissimilar ecologically, as genetics or behaviour are less likely to be well adapted to conditions in the recipient area. Additional logistical difficulties would also be presented by movements over greater distances or between jurisdictions. Captive breeding for release could be a safer option, with the success of the operation at Paradise Park in Cornwall being encouraging, although the difficulties encountered and surmounted there suggest that replication elsewhere may not be straightforward (Burgess et al., 2012). The release element has been successful in Jersey (see Section 5.7: Jersey), but releases in Cornwall have left no genetic impact on the local population (see Section 5.5: England), so release and survival of chough will not necessarily lead to improved genetic health of the population. It is unknown whether the released birds facilitated the persistence of naturally colonising birds by increasing foraging flock size and aiding socialisation or anti-predator vigilance. Much has been learned from the releases in Jersey and Cornwall and, should any releases be attempted in Scotland, further consultation will take place (D. Parish, pers. comm., 2023).
6.6 Provision of nest boxes or shelters to enhance breeding
Nest boxes for chough are used in several locations, especially where there are active ringing projects. However, faeces and associated ammonia can build-up inside boxes and affect legs, wings and tail feathers of the young (E. Bignal, pers. comm. 2023). It may, therefore, be better to use open platforms, perhaps with open trays on top of them to prevent young from straying over the edge. This allows birds to defecate over the side. Boxes can also be attractive to jackdaws and barn owls, resulting in competition for nest sites. Such chough shelters incorporating nesting platforms form a specific agri-environment option in Scotland and nest boxes do so on the Isle of Man. They clearly have the potential to increase breeding density if natural nest sites are limiting and this may be the case in some areas of Wales (A. Cross, pers. comm., 2022), but there have been no formal tests of this possibility: no systematic studies have been conducted to assess effects on densities. The effects of boxes are unlikely to be negative and they can facilitate monitoring, so increasing their use could be a worthwhile option in threatened populations.
6.7 Low-input spring-sown cereal crops producing winter stubbles
Stubbles following low-input crops are a key habitat for farmland granivores, but are mentioned as valuable for chough in several schemes (Section 5: Review of population establishment and conservation management measures). However, their benefits to chough as a food source have not been evaluated. The habitat could potentially provide food all year round, but likely mostly in winter and early spring, when the soil will be accessible. However, stubble management is only relevant where there is arable land-use, which will not be common in most regions where chough are present. At most, this intervention will probably only benefit chough in a few, specific localities and may be viewed as an option to be used when circumstances permit.
6.8 Maintenance of earth/stone banks for chough foraging
Suitable chough foraging habitat does not necessarily require large areas of short grass or bare ground: although grazed vegetation is used predominantly, footpaths and stone-faced earth-banks are also favoured for foraging. As with larger grassland areas, these habitats, if characterised by short sward height and bare patches, are heavily favoured by chough (Roberts, 1983; Meyer, 2001; Whitehead et al., 2005). Management of paths and banks in such a way as to allow chough access to feed at bases and on the tops of banks, e.g. by grazing, or cutting where necessary, could promote chough foraging opportunities. This has been applied within the management regime in Cornwall, via Cornish hedges and earth bank field boundaries, especially where unfenced, but its effects have not been evaluated. Overall, it is possible that such habitats are valuable in bridging resource gaps when primary feeding sites are inaccessible due to disturbance, frost or flooding, for example, but they are unlikely to provide critical resources to support populations.
6.9 Avoidance of disturbance and development during the breeding season
Natural cliff nest sites can be disturbed by climbers, while artificial sites are vulnerable to recreational activities if they are in disused quarries, and to operational disturbance if they are in active quarry sites. It is plausible that nest sites in barns (nest boxes/platforms) are also likely to be prone to sustained disturbance because of human proximity, even though certain individuals/pairs may have become habituated to a certain level of disturbance (Colhoun et al., 2023). From observations of nesting birds in cattle sheds in SW Ireland and Islay, some pairs at least do appear to have become habituated to the continuous close activity of farm machinery, livestock and people (S. Cummins pers. comm., 2021, E. Bignal pers. comm., 2023). Chough eggs were historically prized in egg collections (Bullock et al., 1983) and this potentially remains a direct threat today where the species is rare or declining (as it is with other breeding bird species, because rarity is a driver of value for egg-collectors). Many chough areas are fairly inaccessible and remote from direct human disturbance, i.e., on sea cliffs, so disturbance is probably only an issue in specific locations. There has been no systematic testing of the sensitivity of nesting chough to disturbance, so the benefits of avoiding it are not quantifiably known, but it is obvious that some level of sustained disturbance will be too high for any given nesting pair and effects on single pairs will be more significant in smaller populations. Therefore, taking a precautionary principle in contexts like Islay, where birdwatchers/visitors are a likely source of disturbance (J. Graham pers. comm., 2022), or Cornwall, where the population is very small, would seem sensible. The measures that have been proposed include awareness and activity control in quarries and among climbing groups, limiting public access and diversion of footpaths, or widening of areas through which access is allowed to reduce pressure on nest locations. Awareness of the species’ vulnerability should also be applied to birdwatchers/visitors all year round for both nesting and foraging birds at a site level. Recommendations have to be context-specific, relating to the type of disturbance encountered.
6.10 Unploughed field headlands, untreated with pesticides
As with low-input crop stubbles (see Section 6.7: Low-input spring-sown cereal crops producing winter stubbles), this is arable habitat management that has not been evaluated for benefits to chough. The specific management employed here will determine the habitat type that is created, i.e. a permanently vegetated headland will require mowing or grazing to provide valuable habitat for chough, whereas a one-year fallow is likely to be suitable for a limited period, until the vegetation present grows tall and dense. In all cases, the habitat would be expected to be relatively rich in invertebrate food for the local farming system, although accessibility of the food to chough would depend upon the vegetation structure.
6.11 Provision of advice to land managers
It is well-established in the field of agri-environment that in-person consultation with an adviser leads to better quality management by land managers and more positive environmental outcomes, than simply following a written prescription (Smallshire et al., 2004). To some extent, this may reflect the prevention of ‘cheating’, but farmers typically do not have the ecological expertise to interpret the relative value of different interventions for a desired outcome, or the implications of small deviations from an ideal management approach. There is no reason why this should not apply also to the chough context and it has been suggested specifically for management in Pembrokeshire (Box 5.3 Management approaches in Wales), but it has not been formally evaluated in terms of its effects. Given the chough’s status as a flagship species in many areas, awareness and interest may not need as much prompting as they do for some other targets of environmental management, but investment in advice to tailor interventions to local conditions is nevertheless likely to be effective.
6.12 Anthelmintic treatment of choughs
During 2014-2018, antihelmintic drugs were administered to chough on Islay as a treatment for high parasite loads (in combination with supplementary feeding), with apparently positive effects on survival (Trask et al., 2020; Fenn et al. 2021). However, this type of intervention does not address the ultimate causes of high parasite burden, which are more likely to be poor body condition and genetic homogeneity (Trask et al., 2020), and is also difficult and labour-intensive. Therefore, such treatment interventions are likely only applicable for local, acute issues with gapeworm etc., rather than a long-term, transferable measure.
6.13 Other possible management measures
In principle, any land management practice could be applied as a specific intervention for environmental benefit, but not all can clearly be applied in any given land-use context. Nevertheless, several interventions are suggested by the evidence relating to biotic and abiotic influences (Section 2.2: Review of the evidence for the influences of biotic and abiotic factors (and interactions between them) on chough population dynamics) that could be applied in this way. One key one, from the evidence on Islay, is that of cut silage fields as a foraging habitat. On Islay, newly fledged and sub-adult chough use the flush of insect food in cut silage fields as a resource, as they become available in summer (Reid et al., 2009, Gilbert et al., 2019b). Increasing quantities of silage in the landscape would be likely to have other negative consequences, such as loss of extensive grassland. However, staggering the dates of silage cutting in an area, including some early cutting in June, could extend the period over which freshly-cut silage is available to newly fledged chough considerably, with minimal cost to landowners (if done sensibly). Care would be needed to ensure that significant grass yield is not lost by early cutting and that the operation is not compromised by grass growing too tall and ‘lodging’, but judicious use of one- and two-cut strategies per season should minimise these problems. Note, however, that some payment to farmers may still be necessary to encourage uptake or to pay for income foregone.
Another interesting potential food source to bridge temporal resource gaps is that of insects that are attracted to tideline wrack. Currently, chough exploit this resource opportunistically when weather conditions provide and maintain it, but deliberate operations to dredge for wrack and to leave it just above the tideline, or simply to move naturally deposited wrack above the line where it would be dispersed by subsequent tidal action, could work as an approach to manage the availability of the resource. However, dredging is likely to conflict with other interests, so its viability is likely to be low, regardless of any likely ecological benefits (J. Graham, pers. comm., 2023). In addition, sandhoppers (Order Amphipoda) associated with wrack can act as intermediate hosts for potentially pathogenic internal parasites of choughs, such as gizzard worms and thorny-headed worms (Trask et al., 2020), so food-resource benefits could be outweighed by disease disbenefits.
Finally, the near demise of active shepherding/cow-herding has led to the effective abandonment of areas of upland pastures (J.R. Calladine, pers. comm., 2022), suggesting that similar organisation of grazing effort in coastal areas could provide better control of habitat conditions via a more consistent spatial distribution of grazing pressure. Unsupervised livestock are likely to preferentially forage on more palatable or nutritious vegetation, or in more sheltered areas, and, hence, to generate a more heterogeneous vegetation structure. However, active shepherding could drive them to consume a wider range of vegetation more evenly, and help to deliver a target sward type across a large area in general. This could improve habitat for chough more efficiently than measures such as mechanical scrub removal and spot-spraying.
7. Discussion
Following very long-term, historical declines, chough have been largely stable in the British Isles in recent decades, such that they do not appear on lists of species of conservation concern. However, this masks critical local declines and population challenges at the northern edge of the species’ range, in Scotland, and its loss from Northern Ireland. Chough conservation management and monitoring in the UK, Ireland and Isle of Man has largely been undertaken independently in different regions, with the exception of decadal censuses of the UK and Irish populations. Understandably, targeted conservation and research have largely been carried out where numbers are either declining (e.g., Scotland) or where populations remain particularly vulnerable to a number of threats (e.g., Cornwall). Therefore, there could be a lack of knowledge of the details of chough ecology in other, less well studied, populations that can be translated into positive management measures for the Scottish context.
Nevertheless, a fundamental requirement is the provision of suitable foraging habitat, which is reflected in the evidence and most agri-environment (and other) initiatives that have been prescribed for chough in different regions. Critically, these aim to deliver chough-specific grazing and stocking systems that provide a short sward and bare ground via rather intensive grazing, but with low fertiliser inputs, and prevention of use of certain anti-parasitic drugs for livestock to ensure necessary food resources provided by dung. Supplementary feeding has been of critical value in Scotland, but has not been applied elsewhere, and a need for it has not been identified. In the long term, it will be necessary to replace supplementary feeding with self-sustaining habitat management practices. If the widely recommended grazing management approaches do not suffice, trialling of novel approaches involving tidal wrack, shepherding or silage management could be valuable. There may also be nuances to the management of grassland and grazing that can enhance food resource value for chough: for instance, there are few ground-truthed data on the availability and accessibility of invertebrate prey within habitats that are subject to different management regimes. Understanding these details better may suggest effective revisions to current practices.
The Scottish chough population has seen a steady decline, while most other chough in the UK and Ireland have reported increased or stable populations in recent decades, and there is some evidence of concomitant variation in demography. Therefore, comparing differences in grazing intensity, stocking density and anti-parasitic treatments between these regions could inform further management possibilities. The lower overall stocking rates on Islay, for example, are a likely consequence of a lower potential for livestock production due to economic disadvantages the island farmers have compared with their mainland counterparts (Jonsson et al., 2020). Detailed information comparing grazing management practices between chough populations was not available for this review, but examining their effects on chough demography would be a worthwhile topic for further research, as there is significant variation between regions (Jonsson et al., 2020). However, climatic and grazing differences between grassland used by chough in Cornwall and Islay, for example, may lead to important differences in habitat suitability that mean that results are not transferable. Ecological research involving healthy populations would therefore be valuable, but suggested management approaches from them would still need to be trialled in Scotland, for example.
The focus of much conservation-based research on declining and threatened populations becomes apparent in the relative scarcity of peer-reviewed material relating to the UK (excluding Scotland) and Irish populations. Other than published census results and population updates to annual/bi-annual bird reports and magazine articles, there appear to have been very few investigative peer-reviewed studies carried out on chough in the UK in recent decades. However, this does not imply that these populations do not receive adequate conservation attention. In fact, this review has highlighted the extensive amount of dedicated work (mostly voluntary) and specialist expertise that a number of these chough populations have received in the last 30 years. It is clear that, where possible, annual surveillance of breeding and wintering numbers, together with annual survival rates and breeding success rates, offer a greater level of population monitoring and demographic detail that would otherwise go unreported between censuses. It should also enable conservationists and researchers to become aware of any sudden adverse changes in the discrete populations and react accordingly. However, the various local data collection and monitoring projects are currently not exploited to their fullest and there is clear potential to conduct more formal analyses comparing breeding success and survival rates between regions, and with respect to habitat and management variables that are derived from often-expert local knowledge and remote-sensed observations.
Increasing populations offer potential to investigate ecological relationships that could be relevant to conservation measures to apply elsewhere. For example, a tendency to move into man-made nest sites in Wales and Ireland could reflect necessity as natural sites are filled, or facilitation because nesting opportunities are limiting and this behavioural shift actually makes the increase possible. Then, particularly in the former case, these sites could be higher or lower in quality than natural sites, such that breeding success is improved or reduced. At the extreme, they could be ecological traps, attracting birds to nest but delivering lower success than other available sites. Competition with other species, such as Jackdaw or Stock Dove Columba oenas, could also create issues in some circumstances. The only comparison to date found no difference in average production of young (Bignal et al., 1987), but considered just one location more than 30 years ago. It would be valuable to investigate the implications of the development that, for example, a significant proportion of the population in southwest Ireland now nests in man-made sites (S. Cummins, pers. comm., 2023). Deliberate provision of such sites could become a conservation measure elsewhere if the evidence supports it.
In addition to the habitat and food influences that are critical for chough throughout their range, the Scottish population suffers from significant genetic challenges due to long-term inbreeding, which compromises the long-term sustainability of the population (Trask et al., 2020). While it is important to consider the unique context of the Scottish population, at the northernmost limit of the chough’s global geographical range and possibly in the final stages of a period of decline and range contraction that has lasted for centuries, the relative success of the local populations in Wales and Cornwall, in combination with a warming climate, suggest that a sustainable population that increases over the next few decades is not impossible. Limits to expansion, say northward along the west coast of Scotland, may be in the genetics and behaviour of a relic, remnant population, i.e., the opposite of the recently colonising population context in Cornwall. Boosting the Scottish birds genetically could perhaps both enhance sustainability and promote expansion.
Increasing genetic diversity is, however, difficult. Captive breeding has succeeded in Cornwall, but releases there have had little effect, it seems, although releases in Jersey have succeeded (Mitchell, pers. comm., 2022, Operation Chough, 2021). Lessons need to be learned from both contexts if releases are to be considered in Scotland. However, natural dispersal would be a better way for populations to (re-)establish, if it can occur. Although no long-distance movements of the intensively colour-ringed chough on Islay have been recorded, the Cornish birds show that this is possible, and colour-ringed chough movements have been recorded from North Wales to South Wales (219 km), and to the Isle of Man (c.100km across open sea), from South Wales across the Bristol Channel, and even from Brittany to North Devon (Owain, 2016.). More work on ring-resighting has the potential to reveal more about the key determinants of chough dispersal. However, ways to facilitate movements of dispersing individuals that could potentially found new populations or join existing ones, making such events more common, would be valuable. In addition to aiming to bolster the Islay chough with new genetic stock, this suggests that there would be value in aiming simultaneously to establish other populations at points closer to other existing populations. Such coordinated action, including consideration of the genetics within each ‘stepping stone’ new location, could deliver longer-term sustainability for a northern metapopulation. Suitable establishment locations might be those from which chough have disappeared in the relatively recent past, such as Dumfriesshire and the Northern Irish coast. Management to create suitable habitat alone could be sufficient to attract colonising populations in the medium term, or further releases of captive-bred birds may be required.
8. Conclusions
It is evident that a combination of three main factors has driven the population decline of chough in Scotland: lack of natural food resources, parasite burdens and genetics-related health issues, with parasite problems most probably being a consequence of the other two (Trask et al., 2020). There are no obvious biotic or abiotic external influences that are unique to this population, but the details of the ecology of healthier populations in the British Isles are much less well-studied; detailed comparative ecological research is therefore needed. Clearly, all chough populations in this range share the same basic ecology and management interventions are probably largely transferable, although land-use context, ecological and climatic influences that differ regionally cannot be ruled out. There is some indication of systematic demographic differences between regional populations, but formal analyses of the available data are required.
Successful conservation of the Scottish population is going to depend on habitat management, combined with enhancements of genetic diversity. A combination of established and novel approaches can contribute to the former, while the latter will require captive-breeding and release in the short term and, probably, broader-scale management to enhance population connectivity in the long term. Many of these measures, including the release of captive-bred birds, require development, testing and/or refinement to the specific context in Scotland. However, it remains conceivable that the very long-term context of decline and range contraction for the Scottish population, in addition with their edge-of-range distribution, may present significant challenges to implementing the specific interventions necessary to halt or reverse the current situation.
8.1 Recommendations for future monitoring and research
- Collation and standardised, integrated reporting of the regional monitoring efforts would be hugely valuable, but must be funded in such a way as to support the volunteer groups who collect the data as well as the coordinating body.
- Using these collated (raw) data, analyses of breeding success and survival with respect to habitat and management variation across regions would allow formal comparisons to investigate the drivers of variation.
- Investigation of the detailed feeding ecology of chough in regions with stable populations in order to compare it with the situation in Scotland. This would include identifying how management measures affect invertebrate food resources in different land-use and climatic contexts.
- Research to gauge potential effects of disturbance on breeding and foraging birds - examining demographic effects, alert distances, flight initiation distances and establishing appropriate disturbance buffer parameters to produce workable guidelines.
- Comparative assessment of invertebrate prey availability in soils between populations in different regions: measuring prey abundance and diversity, and the composition of soil types (e.g., moisture levels, pH levels, nutrient levels, contaminants etc.), with respect to land-use and farming systems.
- Trialling of novel management approaches, i.e., management of tidal wrack, landscape-wide coordination of silage cutting and active shepherding, particularly in the post-fledging period, with a view to designing transferable management prescriptions for delivering the resources that are currently provided by supplementary feeding.
- Research into the potential of predation to drive the population changes in British and Irish chough could be conducted, although it should be supported by clear hypotheses and also consider practical solutions. All natural populations are subject to predation, so any conclusion of importance for population change needs to demonstrate an increase in predation pressure over time and a correlation with declines. It is also important to recognise that vulnerability to predation is a function of multiple factors, including habitat and population influences: the ultimate drivers may be unrelated to predators per se. Note that such studies would be challenging logistically to conduct using field sampling, as long time series would probably be needed; hence a modelling approach, using existing data for key demographic parameters and new field data where necessary to fill any gaps, might be most practical. There is no clear hypothesis to test regarding predation impacts on chough.
- An updated, multi-site assessment of breeding success of chough in man-made versus natural nest sites would inform whether the shift to such sites in some areas is a positive or negative development overall and whether it suggests a potential conservation measure.
- Research on the species’ dispersal capabilities, and the factors facilitating them, could help to inform the potential of the Scottish population to expand its breeding range. This would be relevant to predicted northward range expansion from climate predictions. This could include direct measurement of dispersal using targeted colour-ring resighting and/or satellite tagging.
- Regular monitoring, with Irish colleagues, of the recent breeding range contraction in Donegal, as this is the nearest breeding population to the Scottish population and so could have significant implications for naturally dispersing Irish birds that represent potential natural colonists.
- A scoping study to identify potential sites to be managed as suitable for chough as stepping stones between Islay and established populations on the Isle of Man and in Ireland. These could be on Kintyre, Arran, Galloway or coastal Northern Ireland and, ultimately, chough management options and chough introductions could be made there.
- Consultation about and review of chough release procedures, as applied in Cornwall and Jersey, with a view to informing the use of effective approaches in Scotland.
- Further genetic and population viability analyses to develop a strategy for sourcing genetic material to deliver an effective enhancement of the gene pool in the Scottish population by bolstering from specific populations elsewhere (e.g., Irish or Isle of Man populations).
References
Allen and Mellon Environmental Ltd. 2021. National Museums Northern Ireland. Northern Ireland Priority Species. Pyrrhocorax pyrrhocorax – chough. 2006-10.
Ausden, M. and Bateson, D. 2005. Winter cattle grazing to create foraging habitat for choughs Pyrrhocorax pyrrhocorax at South Stack RSPB Reserve, Anglesey, Wales. Conservation Evidence, 2, 26-27.
Baker, D.J., Freeman, S.N., Grice, P.V. and Siriwardena, G.M. 2012. Landscape scale responses of birds to agri-environment management: a test of the English Environmental Stewardship scheme. Journal of Applied Ecology, 49, 871-882.
Balmer, D.E., Gillings, S., Caffrey, B.J., Swann, R.L., Downie, I.S. and Fuller, R.J. 2013. Bird Atlas 2007-11: the breeding and wintering birds of Britain and Ireland. Thetford: BTO.
Baxter, E. V. and Rintoul, L. J. 1953. The Birds of Scotland. Edinburgh: Oliver and Boyd, Edinburgh.
Berrow, S., Mackie, K., O’Sullivan, O., Shepherd, K., Melon, C. and Coveney, J. 1993. The second international chough survey in Ireland. Irish Birds, 5, pp.1-10.
Beynon, S., Peck, M., Mann, D. and Owen, L. 2012. Consequences of alternative and conventional endoparasite control in cattle for dung-associated invertebrates and ecosystem functioning. Agriculture, Ecosystems and Environment, 162, 36–44.
Bignal, C. and Bignal, E. 2011. Supplementary feeding of sub-adult choughs. British Wildlife, 22 (5).
Bignal, E. 2021. Conservation management of red-billed choughs in Scotland: Supplementary feeding and demographic monitoring 2020 -2022: Interim report 2.
Bignal, E., Bignal, S. and Curtis, D.J. 1989. Functional unit systems and support ground for choughs – the nature conservation requirements. In: E. Bignal and D.J. Curtis, Eds. Proceedings of the International Workshop on the Conservation of the Chough, Pyrrhocorax pyrrhocorax, in the EC. 11-14 November 1988. SCSG.
Bignal, E., Monaghan, P., Benn, S., Bignal, S., Still, E. and Thompson, P. 1987. Breeding success and post-fledging survival in the chough Pyrrhocorax pyrrhocorax. Bird Study, 34, 39-42.
Birdlife International/European Bird Census Council. 2000. European Bird Populations: estimates and trends. Birdlife International. Cambridge.
BirdLife International, 2016. Pyrrhocorax pyrrhocorax. The IUCN Red List of Threatened Species 2016: e.T22705916A87384853.
Birkhead, T.R. 1991. The Magpies: The ecology and behaviour of black-billed and yellow-billed magpies. Poyser, London, 135-136.
Birds on the Edge, 2014a. Restoration of the red-billed chough in Jersey.
Birds on the Edge, 2014b. Reducing bracken coverage on Jersey's coastal lands.
Birds on the Edge, 2014c. Returning livestock grazing to Jersey's coasts.
Bossema, I., Roell, A. and Baeyens, G. 1986. Adaptations to inter-specific competition in five corvid species in the Netherlands. Ardea, 74, 199-210.
Brak, B.H., Hilarides, L., Elbersen, B.S., and van Wingerden, W.K. 2004. Extensive livestock systems and biodiversity: The case of Islay. Alterra.
Bullock, I.D., Drewett, D.R. and Mickleburg, S.P. 1983. The chough in Britain and Ireland. British Birds, 76, 377-401.
Burgess, M.D., Woolcock, D., Hales, R.B., Waite, R. and Hales, A.J. 2012. Captive Husbandry and Socialization of the red‐billed chough (Pyrrhocorax pyrrhocorax). Zoo Biology, 31(6), 725-735.
Carter, I., Brown, A., Lock, L., Wotton, S. and Croft, S., 2003. The restoration of the red-billed chough in Cornwall. British Birds, 96(1), 23-29.
CBWPS (Cornwall Bird Watching and Preservation Society), 2022. Cornish choughs: 2022 Season update.
Chamberlain, D., Gough, S., Anderson, G., Macdonald, M., Grice, P., and Vickery, J. 2009. Bird use of cultivated fallow ‘Lapwing plots’ within English agri‐environment schemes. Bird Study, 56(3), 289-297.
Clarke, J. & Clarke, P.M. 1995. Choughs feeding on Mining Bee Colletes succinctus larvae on Colonsay. Bird Study, 42(3), 253-254.
Colhourn, K., Rooney, E., Collins, J., Keogh, N., Lauder, A., Heardman, C. and Cummins, S. 2023. Status and Distribution of Chough in Ireland: Results of the national survey 2021. National Parks and Wildlife Service. ISSN 1393 – 6670.
Cook, A.S., Grant M.C., McKay C.R. and Peacock M.A. 2001. Status, distribution and breeding success of the red-billed chough in Scotland in 2002. Scottish Birds, 24, 11-17.
Coombs, F. 1978. The Crows. A study of the Corvids of Europe. B.T. Batsford, London.
Corry, E. 2021. Chough report: April 2021. Birds on the Edge.
Corry, E., Jones, C., Hales A. and Young G. 2021. Reintroduction of the red-billed chough in Jersey, British Channel Islands. IUCN Global conservation translocation perspectives: Case studies from around the globe, 103-107.
Cramp, S., Perrins, C.M. and Brooks, D.J. 1994. Handbook of the Birds of Europe, the Middle East and North Africa. The Birds of the Western Palearctic. Vol. 8. Oxford University Press.
Cross, A.V., Stratford, A., Johnstone, I., Thorpe, R.I.T., Dodd, S., Peach, W., Buchanan, G. and Moorhouse-Gann, R. 2020. Red-billed Chough Wales research programme. Unpublished Natural Resources Wales Science Report.
DAFM (Department of Agriculture Food and the Marine), 2020. Released GLAS Specification 2015.
DEFRA (Department for Environment Food and Rural Affairs), 2023. Environmental land management schemes: details of actions and payments.
Dustow, J. 2020. Chough summary report (unpublished).
Eaton, M.A., Brown, A.F., Noble D.G., Musgrove, A.J., Hearn, R., Aebischer, N.J., Gibbons D.W., Evans, A. and Gregory R.D. 2009. Birds of Conservation Concern 3: the population status of birds in the United Kingdom, Channel Islands and the Isle of Man. British Birds, 102, 296–341.
Fenn, S.R., Bignal, E.M., Trask, A.E., McCracken, D.I., Monaghan, P., Reid, J.M. 2020. Collateral benefits of targeted supplementary feeding on demography and growth rate of a threatened population. Journal of Applied Ecology, 57, 2212–2221.
Fenn, S. R., Bignal, E. M., Bignal, S., Trask, A. E., McCracken, D. I., Monaghan, P., & Reid, J. M. 2021. Within-year and among-year variation in impacts of targeted conservation management on juvenile survival in a threatened population. Journal of Applied Ecology, 58, 2722–2733.
Finney, S.K. and Jardine, D.C. 2003. The distribution and status of the chough in Scotland in 2002. Scottish Birds, 24, 11-17.
Forrester, R., Andrews, I., McInerny, C., Murray, R., McGowan, R.Y., Zonfrillo, B., Betts, M., Jardine, D. and Grundy, D. 2007. The birds of Scotland. Scottish Ornithologist's Club.
Gilbert, G., MacGillivray, F.S., McKay, C.R. and Robertson, H.S. 2019a. Foraging habitat of a declining Scottish red-billed chough Pyrrhocorax pyrrhocorax population in the post-breeding period. Bird Study, 66(1), 32-42.
Gilbert, G., MacGillivray, F.S., Robertson, H.L. and Jonsson, N.N. 2019b. Adverse effects of routine bovine health treatments containing triclabendazole and synthetic pyrethroids on the abundance of dipteran larvae in bovine faeces. Scientific Reports, 9(1), 1-10.
Gilbert, G., Stanbury, A. and Lewis, L. 2021. Birds of conservation concern in Ireland 4: 2020–2026. Irish Birds, 43, 1-22.
Government of Ireland. 2021. Released GLAS Specification. Conservation of Farmland Birds; Chough, 19.
Gray, N., Thomas, G., Trewby, M. and Newton, S.F. 2003. Pyrrhocorax pyrrhocorax in the Republic. Irish Birds, 7, 147-156.
Green, M. and Williams, I. 1992. The status of the chough Pyrrhocorax pyrrhocorax in Wales. Welsh Birds, 6, 77-84.
Haycock, R. J., Hodges, J. E. and Johnstone, I. 2021. Long-term patterns in Red-billed Chough territory occupation and breeding performance in Pembrokeshire. Report No: 634. Report to Natural Resources Wales.
Hales, R. 2021. Operation Chough: Operation Chough Timeline.
Hayhow, D.B., Johnstone, I., Moore, A.S., Mucklow, C., Stratford, A., Šúr, M. and Eaton, M.A. 2018. Breeding status of red-billed choughs Pyrrhocorax pyrrhocorax in the UK and Isle of Man in 2014. Bird Study, 65(4), 458-470.
Hodges, J.E. 1994. A Chough Conservation Strategy for Pembrokeshire, Pembrokeshire Coast National Park.
Hodges, J. E. and Haycock, R. J. 2020. Annual surveillance of choughs in the Pembrokeshire Coast National Park 1992-2020. Unpublished reports CCW/NRW, PCNPA, and Pembrokeshire Chough Study Group.
Hudson, G. and Henderson, D.J. 1983. Soils of the Inner Hebrides. Cambridge University Press.
Hughes, J. 2020. Welsh Bird Report 2019. Birds in Wales, 17(3).
Humphrey, K. and Murphy, J. 2017. UK Climate Change Risk Assessment Evidence Report. Report prepared for the Adaptaion Sub-Committee of the Committee on Climate Change, London.
Isle of Man Government. 2008. Guidelines for the selection of biological Areas of Special Scientific Interest (ASSIs) on the Isle of Man. Volume 2 DAFF.
Jardine, D., Peacock, M., Vaughan, M. and Fisher, I. 2019. Choughs on Colonsay and Oronsay, 1984-2018. British Birds, 112, 390-398.
Johnstone, I., Thorpe, R., Moore, A. and Finney, S. 2007. Breeding status of choughs Pyrrhocorax pyrrhocorax in the UK and Isle of Man in 2002. Bird Study, 54(1), 23-34.
Jonsson, N.N., Gilbert, G. and MacGillivray, F.S. 2020. Livestock management in red-billed chough feeding habitat in Great Britain and the Isle of Man. Rangeland Ecology and Management, 73(2), 216-223.
Kent Wildlife Trust. 2021. Restoring the Chough to Kent.
Kerbiriou, C. and Le Viol, I. 1999. Landscape management for chough Porz Gwenn (Isle of Ouessant, France). In: Choughs and Farming Seminar Abstracts, Islay. RSPB.
Lamacraft, D. 2004. Species Action Plan for Chough, RSPB Cymru.
Lawson, B., Robinson, R.A., Colvile, K.M., Peck, K.M., Chantrey, J., Pennycott, T.W., Simpson, V.R., Toms, M.P. and Cunningham, A.A. 2012. The emergence and spread of finch trichomonosis in the British Isles. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1604), 2852-2863.
Loder, J. de V. 1935. Colonsay and Oronsay in the Isles of Argyll: their history, flora, fauna and topography. Oliver and Boyd. Edinburgh and London.
MacDonald, M.A., Angell, R., Dines, T.D., Dodd, S., Haysom, K.A., Hobson, R., Johnstone, I.G., Matthews, V., Morris, A.J., Parry, R., Shellswell, C.H., Skates, J., Tordoff, G.M. and Wilberforce, E.M. 2019. Have Welsh agri‐environment schemes delivered for focal species? Results from a comprehensive monitoring programme. Journal of Applied Ecology, 56(4), 812-823.
MacGillivray, F.S., Gilbert, G., & McKay, C.R. 2018. The diet of a declining red-billed chough Pyrrhocorax pyrrhocorax population on Islay, Scotland. Bird Study, 65(3), 422-425.
McCanch, N. 2000. The relationship between red-Billed chough Pyrrhocorax pyrrhocorax breeding populations and grazing pressure on the Calf of Man. Bird Study, 47(3), 295-303.
McCracken, D.I. 1990. Factors affecting the availability of invertebrate food for the chough, Pyrrhocorax pyrrhocorax. PhD thesis, University of Glasgow.
McCracken, D.I. and Foster, G.N. 1993. The effect of ivermectin on the invertebrate fauna associated with cow dung. Environmental Toxicology and Chemistry, 12, 73–84.
McKay, C. 2007. Red-billed Chough. In Birds of Scotland (Eds Forrester, R.W. & Andrews, I.A.). Scottish Ornithologists’ Club, Aberlady.
McKay, C.R. 1996. Conservation and ecology of the red-billed chough Pyrrhocorax pyrrhocorax. PhD thesis.
Meyer, R.M. 1991. The feeding ecology of the red-billed chough Pyrrhocorax pyrrhococorax L. in west Wales, and the feasibility of re-establishment in Cornwall. PhD thesis.
Monaghan, P., Bignal, E., Bignal, S., Easterbee, N. and McKay, C.R. 1989. The distribution and status of the chough in Scotland in 1986. Scottish Birds, 15, 114-118.
Moore, A.S. 2004. Choughs on the Isle of Man in 2002. Peregrine, 8(4), 338.
Moore, A.S. 2005. Choughs on the Isle of Man in 2002. Peregrine, 9(2), 333.
Moore, A.S. 2006. Choughs in the Isle of Man in 2005. Peregrine, 9(10), 146-152.
Moore, A.S. 2008. Choughs in the Isle of Man in 2007. Peregrine, 9(4), 329-333.
Moore, A.S. 2010. Choughs on the Isle of Man in 2009. Peregrine, 10(2), 134.
Moore, A.S. 2015. Choughs on the Isle of Man in 2014. Peregrine, 10(3), 330.
Moore, A.S. 2016. Choughs on the Isle of Man in 2013. Peregrine, 10, 467.
Moore, A.S. 2017. Choughs in the Isle of Man in 2014. Peregrine, 10(5), 591-593.
Moore, A.S. 2018. The Manx Chough census of 2014 and 2015. Peregrine, 10(6), 702-711.
Moore, A.S. 2020. Choughs in the Isle of Man in 2016. Peregrine, 11, 97-103.
Newbery, P. 1998. Species Action Plan 1559 Chough Pyrrhocorax pyrrhocorax. RSPB unpublished report.
Newton, I. 1994. Experiments on the limitation of bird breeding densities: a review. Ibis,136, 397- 411.
Northern Ireland Rural Development Programme, 2007. Countryside Management Scheme 2007-2013.
Operation Chough, 2021. Welcome to Operation Chough.
Owain, G. 2016. Colour-ringed Choughs in Wales: how and what re-sighting data to report. Gower Ringing Group.
Owen, D.A.L. 1985. Factors affecting the status of the chough in Britain, with observations on its behaviour. PhD thesis.
Owen, D.A.L. 1989. Factors affecting the status of the Chough in England & Wales 1780–1980. In Choughs and Land-use in Europe, Proceedings of an International Workshop on the Conservation of the Chough Pyrrhocorax pyrrhocorax in the EC 11–14 November 1988, pp. 72–80. Scottish Chough Study Group, Argyll.
Pennant, T. 1774. A Tour in Scotland and Voyage to the Hebrides, 1772. John Monk, Chester.
Ratcliffe, D. A. 1980. The Peregrine Falcon. Poyser, Calton
Reid, J.M., Bignal, E., Bignal, S., McCracken, D.I., Bogdanova, M.I. and Monaghan, P. 2008. Investigating patterns and processes of demographic variation: environmental correlates of pre-breeding survival in red-billed choughs (Pyrrhocorrax pyrrhocorax). Journal of Animal Ecology, 77, 777-789.
Reid, J.M., Bignal, E.M., Bignal, S., McCracken, D.I. and Monaghan, P. 2003. Environmental variability, life-history covariation and cohort effects in red-billed choughs Pyrrhocorax pyrrhocorax. Journal of Animal Ecology, 72, 36- 46.
Reid, J.M., Bogdanova, M.I., Bignal, E.M., Bignal, S., McCracken, D.I. and Monaghan, P. 2009. Population ecology and conservation of red-billed choughs in Scotland. Final report on Knowledge Transfer Project.
Reid, J.M., Bignal, E.M., Bignal, S., Bogdanova, M.I., Monaghan, P. and McCracken, D. I. 2011. Diagnosing the timing of demographic bottlenecks: sub-adult survival in red-billed choughs. Journal of Applied Ecology, 48, 797–805.
Richner, H. 1990. Helpers-at-the-nest in carrion crows Corvus corone corone. Ibis, 132(1), 105-108.
Roberts, P. J. 1983. Feeding habitats of the chough on Bardsey Island (Gwynedd), Bird Study, 30(1), 67-72.
Roberts, J. L. and Hawkins, J. 1990. Incompatibility of Choughs and Peregrines. British Birds, 83, 288.
Robinson, R. A. 2005. BirdFacts: profiles of birds occurring in Britain & Ireland. BTO, Thetford.
Rolfe, R. 1996. The status of the chough in the British Isles. Bird Study, 13, 221-236.
RSPB, 2021. Cornwall Chough Project, RSPB.
Rural and Environmental Science and Analytical Services Division, Scottish Government. 2021. June Agricultural Census 2021.
Rylands, K., Mucklow, C. and Lock, L. 2012. Management for choughs and coastal biodiversity in Cornwall: the need for grazing. RSPB
Siriwardena, G.M., Baillie, S.R., Crick, H.Q.P., Wilson, J.D. and Gates S. 2000. The demography of lowland farmland birds. In: Aebischer, N.J., Evans, A.D., Grice, P.V. and Vickery, J.A. eds. Ecology and Conservation of Lowland Farmland Birds, 117-133. Tring: British Ornithologists’ Union.
Siriwardena, G.M., Calbrade, N.A. and Vickery, J.A. 2008. Farmland birds and late winter food: does seed supply fail to meet demand? Ibis, 150, 585–595.
Smallshire, D., Robertson, P. and Thompson, P. 2004. Policy into practice: the development and delivery of agri‐environment schemes and supporting advice in England. Ibis, 146, 250-258.
Soil Survey of Scotland Staff, 1988. Land Capability for Forestry of Scotland at a Scale of 1:250 000. Macaulay Land Use Research Institute, Aberdeen.
Stanbury, A., Eaton, M., Aebischer, N., Balmer, D., Brown, A., Douse, A., Lindley, P., McCulloch, N., Noble, D. and Win, I. 2021. The status of our birds. British Birds, 114, 723-747.
Still, E.A. 1989. Socio-ecology of non-breeding choughs Pyrrhocorax pyrrhocorax. PhD thesis.
Strong, L., and Brown, T. 1987. Avermectins in insect control and biology: a review. Bulletin of Entomological Research, 77(3), 357-389.
Thom, V.M. 2010. Birds in Scotland. T and AD Poyser.
Trask, A.E., Bignal, E.M., McCracken, D.I., Monaghan, P., Piertney, S.B., and Reid, J.M. 2016. Evidence of the phenotypic expression of a lethal recessive allele under inbreeding in a wild population of conservation concern. Journal of Animal Ecology, 85(4), 879-891.
Trask, A.E., Bignal, E., Fenn, S.R., McCracken, D.I., Monaghan, P. and Reid, J.M. 2020. Conservation strategy for red-billed choughs in Scotland: Assessment of the impact of supplementary feeding and evaluation of future management strategies. Scottish Natural Heritage Research Report No. 1152.
Tucker, G. M. 1992. Effects of Agricultural Practices on Field Use by Invertebrate-Feeding Birds in Winter. Journal of Applied Ecology, 29(3), 779–790.
Warnes, J. and Stroud, D.A. 1989. Habitat use and the diet of chough on the island of Islay Scotland. Choughs and land-use in Europe. Proceedings of an international workshop on the conservation of the chough, Pyrrhocorax pyrrhocorax, in the EC.
Webb, L., McCracken, D., Beaumont, D. and Nager, R. 2006. Project Information Note: Conservation considerations regarding the use of avermectin animal health products. RSPB, Edinburgh, SAC, Edinburgh and University of Glasgow, Glasgow.
Welsh Government. 2020. Glastir Advanced 2018 Rules Booklet 2: Whole Farm Code and Management Options The Welsh Government Rural Communities – Rural Development Programme for Wales 2014-2020.
Welsh Government, 2021. Species in Focus: Chough. Pori Natur a Threftadaeth.
Wenzel, M.A., Webster, L.M., Blanco, G., Burgess, M.D., Kerbiriou, C., Segelbacher, G., Piertney, S.B. and Reid, J.M. 2012. Pronounced genetic structure and low genetic diversity in European red-billed chough (Pyrrhocorax pyrrhocorax) populations. Conservation Genetics, 13(5), 1213-1230.
Wenzel, M.A., Webster, L.M., Blanco, G., Burgess, M.D., Kerbiriou, C., Segelbacher, G., Piertney, S.B. and Reid, J.M. 2015. Erratum to: Pronounced genetic structure and low genetic diversity in European red-billed chough (Pyrrhocorax pyrrhocorax) populations. Conservation Genetics, 16(4), 1011-1012.
Whitehead, S., Johnstone, I. and Wilson, J. 2005. Choughs Pyrrhocorax pyrrhocorax breeding in Wales select foraging habitat at different spatial scales. Bird Study, 52, 193-203.
Yalden, D.W. and Abarella, U. 2009. The History of British Birds. Oxford University press.
Annex
Table A1. Annual breeding success on Islay (taken from Trask et al., 2020)
|
Year |
No. of nests |
No. successful |
Fledglings per pair Including zeroes |
Fledglings per pair Excluding zeroes |
|---|---|---|---|---|
|
2018 |
60 |
26 |
1.23 |
2.85 |
|
2017 |
60 |
29 |
1.18 |
2.40 |
|
2016 |
58 |
29 |
1.21 |
2.40 |
|
2015 |
57 |
30 |
1.21 |
2.30 |
|
2014 |
66 |
44 |
1.42 |
2.14 |
|
2013 |
63 |
36 |
1.06 |
1.78 |
|
2012 |
63 |
33 |
1.38 |
2.64 |
|
2011 |
60 |
28 |
1.35 |
2.89 |
|
2010 |
51 |
32 |
1.61 |
2.56 |
|
2019 |
64 |
31 |
2.06 |
2.53 |
|
2020 |
58 |
19 |
1.55 |
2.36 |
|
2010-2020 |
660 |
337 |
1.39 |
2.41 |
Table A2. Annual breeding success on Colonsay (taken from Jardine et al., 2019)
|
Year |
Total nests |
No. of Fledglings (including zeroes) |
SE |
|---|---|---|---|
|
1990 |
6 |
2.67 |
0.33 |
|
1991 |
9 |
1.78 |
0.40 |
|
1992 |
9 |
2.44 |
0.47 |
|
1993 |
10 |
1.20 |
0.29 |
|
1994 |
7 |
1.71 |
0.64 |
|
1995 |
5 |
2.00 |
0.71 |
|
1996 |
4 |
2.00 |
0.92 |
|
1997 |
8 |
2.00 |
0.53 |
|
1998 |
10 |
2.50 |
0.45 |
|
1999 |
8 |
2.75 |
0.68 |
|
2000 |
5 |
1.20 |
0.80 |
|
2001 |
12 |
2.33 |
0.33 |
|
2002 |
13 |
2.31 |
0.41 |
|
2003 |
17 |
1.12 |
0.32 |
|
2004 |
14 |
2.71 |
0.34 |
|
2005 |
14 |
2.29 |
0.32 |
|
2006 |
17 |
1.94 |
0.34 |
|
2007 |
16 |
1.94 |
0.34 |
|
2008 |
10 |
1.50 |
0.45 |
|
2009 |
11 |
2.45 |
0.21 |
|
2010 |
12 |
1.92 |
0.42 |
|
2011 |
10 |
1.60 |
0.43 |
|
2012 |
11 |
1.91 |
0.49 |
|
2013 |
10 |
1.90 |
0.28 |
|
2014 |
10 |
1.80 |
0.51 |
|
2015 |
10 |
1.60 |
0.51 |
|
2016 |
9 |
2.00 |
0.37 |
|
2017 |
7 |
1.86 |
0.52 |
|
2018 |
5 |
2.00 |
0.00 |
Table A3. Annual breeding success summary data for the Isle of Man, 2002-2016
|
Year |
Including zeroes - Total nests |
Including zeroes - No. of Fledglings |
Including zeroes - SE |
Excluding zeroes - Total nests |
Excluding zeroes - No. of Fledglings |
Excluding zeroes - SE |
|---|---|---|---|---|---|---|
|
2002 |
46 |
1.87 |
1.49 |
42 |
2.05 |
1.61 |
|
2004 |
52 |
2.35 |
1.43 |
50 |
2.44 |
1.48 |
|
2005 |
40 |
2.03 |
1.00 |
32 |
2.53 |
0.83 |
|
2006 |
41 |
1.90 |
1.40 |
36 |
2.17 |
1.52 |
|
2009 |
32 |
1.69 |
1.01 |
26 |
2.08 |
0.99 |
|
2010 |
37 |
2.11 |
1.10 |
35 |
2.23 |
1.14 |
|
2011 |
35 |
2.14 |
1.09 |
31 |
2.42 |
1.12 |
|
2012 |
35 |
1.83 |
0.96 |
28 |
2.29 |
0.83 |
|
2013 |
37 |
1.89 |
1.39 |
33 |
2.12 |
1.51 |
|
2014 |
33 |
1.76 |
1.21 |
29 |
2.00 |
1.31 |
|
2015 |
42 |
1.74 |
1.30 |
35 |
2.09 |
1.39 |
|
2016 |
29 |
2.00 |
0.75 |
24 |
2.42 |
0.52 |
|
Overall |
459 |
1.95 |
0.35 |
401 |
2.24 |
0.37 |
(Moore, 2004, 2005, 2006, 2008, 2010, 2015, 2016, 2017, 2018)
Table A4. Nesting attempts that successfully fledged young from 1992 to 2020, for the Gower and Pembrokeshire populations, as reported in Welsh Bird Reports
Complete data are shown graphically in Haycock et al. (2021); all published information is included here. Young/nest calculated from successful nests only or from all nests when these numbers were not available (*). Mean values calculated from the former only (and all nests in parentheses).
|
Region |
Year |
Occupied territories |
Nests |
Nests from which young fledged |
Min. no of young fledged |
Young fledged/ successful nest |
|---|---|---|---|---|---|---|
|
Gower |
1992 |
1 |
1 |
- |
? |
? |
|
- |
1993 |
? |
- |
- |
- |
- |
|
- |
1994 |
1 |
1 |
- |
4 |
4.00* |
|
- |
1995 |
0 |
- |
- |
- |
- |
|
- |
1996 |
0 |
- |
- |
- |
- |
|
- |
1997 |
0 |
- |
- |
- |
- |
|
- |
1998 |
0 |
- |
- |
- |
- |
|
- |
1999 |
0 |
- |
- |
- |
- |
|
- |
2000 |
1+ |
1+ |
- |
0 |
0.00* |
|
- |
2001 |
2+ |
- |
- |
- |
? |
|
- |
2002 |
1+ |
1+ |
- |
1 |
1.00* |
|
- |
2003 |
1+ |
1+ |
- |
3 |
3.00* |
|
- |
2004 |
3+ |
2+ |
- |
5 |
2.50* |
|
- |
2005 |
2+ |
1+ |
- |
4 |
4.00* |
|
- |
2006 |
6+ |
2+ |
- |
3 |
1.50* |
|
- |
2007 |
8 |
7+ |
- |
16 |
2.29* |
|
- |
2008 |
5 |
4+ |
4 |
9 |
2.25 |
|
- |
2009 |
8 |
8? |
- |
19 |
2.38* |
|
- |
2010 |
6+ |
6? |
- |
- |
? |
|
- |
2011 |
3+ |
3+ |
3 |
7 |
2.33 |
|
- |
2012 |
5 |
5 |
- |
- |
? |
|
- |
2013 |
2+ |
2+ |
2+ |
6 |
3.00 |
|
- |
2014 |
? |
- |
- |
- |
? |
|
- |
2015 |
2 |
2 |
- |
- |
? |
|
- |
2016 |
2 |
2 |
1 |
3 |
3.00 |
|
- |
2017 |
2 |
2 |
- |
- |
? |
|
- |
2018 |
3 |
3 |
2 |
? |
? |
|
- |
2019 |
5 |
2 |
2 |
5 |
2.50 |
|
- |
2020 |
4 |
7 |
2 |
4 |
2.00 |
|
- |
Mean |
- |
- |
- |
- |
2.51 (2.38) |
|
Pembrokeshire |
1992 |
53 |
? |
- |
? |
? |
|
- |
1993 |
? |
- |
- |
? |
? |
|
- |
1994 |
56 |
52 |
- |
83 |
1.60* |
|
- |
1995 |
52 |
38 |
- |
100 |
2.63* |
|
- |
1996 |
52 |
46 |
- |
95 |
2.07* |
|
- |
1997 |
50 |
45 |
- |
108 |
2.40* |
|
- |
1998 |
? |
- |
- |
- |
? |
|
- |
1999 |
55 |
45 |
- |
120 |
2.67* |
|
- |
2000 |
59 |
- |
- |
92 |
1.56* |
|
- |
2001 |
? |
- |
- |
- |
? |
|
- |
2002 |
? |
- |
- |
- |
? |
|
- |
2003 |
64 |
49 |
- |
120 |
2.45* |
|
- |
2004 |
56 |
44(+4) |
- |
83 |
1.89** |
|
- |
2005 |
82 |
81 |
- |
165 |
2.04* |
|
- |
2006 |
68 |
58 |
50 |
111 |
1.91 |
|
- |
2007 |
77 |
52 |
- |
141 |
2.71* |
|
- |
2008 |
73 |
70 |
- |
? |
? |
|
- |
2009 |
74 |
70 |
- |
128 |
1.83* |
|
- |
2010 |
74 |
62 |
- |
114 |
1.84* |
|
- |
2011 |
65 |
59 |
57 |
126 |
2.21 |
|
- |
2012 |
68 |
62 |
41 |
106 |
2.58 |
|
- |
2013 |
65 |
- |
40 |
113 |
2.83 |
|
- |
2014 |
67 |
61 |
40 |
113 |
2.83 |
|
- |
2015 |
74 |
65 |
44 |
118 |
2.68 |
|
- |
2016 |
84 |
76 |
58 |
144 |
2.48 |
|
- |
2017 |
79 |
66 |
51 |
127 |
2.50 |
|
- |
2018 |
77 |
70 |
46 |
116 |
2.50 |
|
- |
2019 |
78 |
72 |
60 |
157 |
2.60 |
|
- |
2020 |
73 |
70 |
61 |
169 |
2.80 |
|
- |
Mean |
- |
- |
- |
- |
2.54 (2.33) |
Table A5. Annual breeding success in Cornwall, England, following the reappearance of a pair in 2001.
|
Year |
Number of Pairs |
Number of successful nests |
Number of chicks fledged |
Chicks per nest excluding zeroes |
Chicks per pair including zeroes |
|---|---|---|---|---|---|
|
2002 |
1 |
1 |
3 |
3.00 |
3.00 |
|
2003 |
1 |
1 |
3 |
3.00 |
3.00 |
|
2004 |
2 |
1 |
4 |
4.00 |
2.00 |
|
2005 |
1 |
1 |
5 |
5.00 |
5.00 |
|
2006 |
3 |
2 |
8 |
4.00 |
2.67 |
|
2007 |
3 |
2 |
9 |
4.50 |
3.00 |
|
2008 |
4 |
2 |
6 |
3.00 |
1.50 |
|
2009 |
7 |
2 |
6 |
4.00 |
0.86 |
|
2010 |
8 |
3 |
9 |
3.00 |
1.13 |
|
2011 |
6 |
4 |
15 |
3.75 |
2.50 |
|
2012 |
6 |
5 |
18 |
3.60 |
3.00 |
|
2013 |
8 |
4 |
13 |
3.25 |
1.63 |
|
2014 |
9 |
5 |
17 |
3.20 |
1.89 |
|
2015 |
9 |
6 |
13 |
2.50 |
1.44 |
|
2016 |
11 |
8 |
23 |
2.88 |
2.09 |
|
2017 |
12 |
6 |
14 |
2.33 |
1.17 |
|
2018 |
19 |
10 |
28 |
2.80 |
1.47 |
|
2019 |
25 |
12 |
38 |
3.25 |
1.52 |
|
2020 |
28 |
14 |
43 |
3.07 |
1.54 |
|
2021 |
41 |
23 |
66 |
2.87 |
1.61 |
(CBPS, 2021)



